Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Saul Moreno and Version 2 by Beatrix Zheng.
Phase change materials (PCMs) are often used to mitigate and time-shift thermal load peaks. They gain heat (charge) during warmer daytime via melting and solidification due to releasing energy (discharge) during cooler nighttime. Therefore, PCMs may increase energy efficiency when integrated into a building’s envelope. They also are used for solar thermal applications such as solar collectors, solar photovoltaic systems, and solar desalination systems.
- phase change material
- buildings
- solar energy
- metal foam
- nano-enhanced
- fin
- PCM
1. Introduction
World energy demand grows as the global population increases. The use of renewable energy has been necessary to cater to this demand and to achieve the seventh Sustainable Development Goal, set up in 2015 by the United Nations General Assembly [1].
The primary renewable energy sources are solar, wind, hydropower, biomass, and tidal. The sun provides all the energy that humanity needs in one hour for one year [2]. Therefore, solar energy is far more abundant than any other energy resource on earth, whether renewable or non-renewable, but it has the disadvantage of being intermittent. In order to address this intermittence, thermal energy storage has been proposed.
The thermal energy storage strategies may be classified into three major groups. They are (a) sensible heat storage, (b) thermochemical heat storage, and (c) latent heat storage. Sensible heat storage is the simplest way to store energy. It consists of a material whose temperature increases/decreases in the energy absorption/release process. Typical materials for sensible heat storage are solids such as sand, ceramic, graphene, rocks, and concrete and fluids such as water, oils, and molten salts [3]. The amount of energy stored is determined by the specific heat capacity of the material, the variation in temperature, and the amount of material. Thermochemical storage uses reversible chemical reactions to store energy. An endothermic reaction charges the storage unit; later, an exothermic reaction discharges it. Latent heat storage is the result of the phase change phenomenon. This storage has a more significant energy storage density than sensible heat storage [4]. The materials to achieve this storage will be described next.
In thermodynamics, the phase change is the transition from one state of matter (solid, liquid, gas, and plasma) to another. The fundamental transitions for each state are shown in Figure 1. The phase change occurs when sufficient energy is supplied/lost by the system. In Figure 1, the phase transitions that require energy are in red, while those that release energy are in blue.
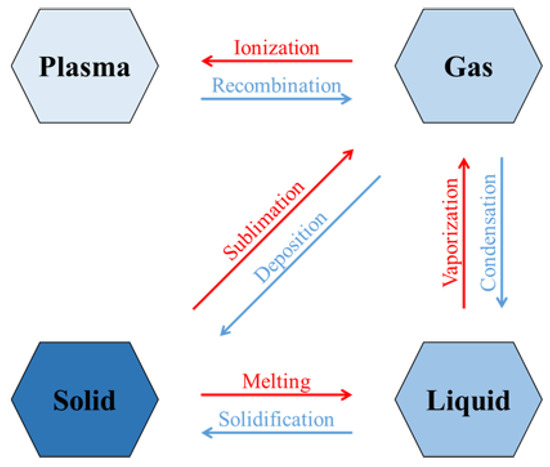
Figure 1.
Phase change transitions.
Scientists have shown particular interest in storing thermal energy in the phase change between solid and liquid. This phase change exhibits certain advantages, such as favorable phase equilibrium, high density, minor volume changes during phase transition, and low vapor pressure at the operation temperature [5].
Javadi et al. [6] reported that the most implemented phase change materials (PCMs) are paraffin waxes, salt hydrates, fatty acids, and eutectic organic/non-organic compounds. These PCMs are classified into three major groups: organic, inorganic (salt hydrates and metallic), and eutectic. The three groups of PCMs have advantages and disadvantages regarding their thermophysical properties. However, organic materials melt congruently without phase separation but have low thermal conductivity. However, it has been observed that paraffin wax is the most widely used organic PCM due to its narrow melting temperature range, which ranges from −10 °C to 67 °C.
On the other hand, inorganic materials stand out for conserving their thermophysical properties when subjected to many thermal cycles and having a much higher thermal conductivity than organic PCMs and a high volumetric latent heat density (~350 MJ/m3). However, the main disadvantages of these PCMs are the corrosiveness of the salts on metals, the subcooling condition, and phase segregation. Finally, eutectic PCMs have a defined melting/freezing point and no component separation during phase changes.
The most important parameter for selecting a PCM for a specific application is its melting point. If the melting point does not coincide with the operating temperature range of the particular application, the phase change phenomenon will not occur. Other relevant parameters are the heat of fusion, thermal and chemical stability, low phase change expansion and contraction, and nontoxicity. Moreover, their availability and low cost are essential for selecting the right PCM.
However, the use of PCMs is broad. The distribution of the PCMs utilized in different low-temperature applications is shown in Figure 2.
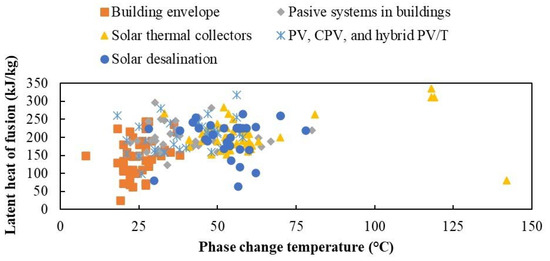
Figure 2.
Phase change temperature and latent heat of fusion in low-temperature applications reported in the literature.
2. Techniques for Improving Heat Transfer in PCMs Systems
From above, the most utilized PCMs for low-temperature applications are the organic solid–liquid ones. However, they have some disadvantages, too, such as low thermal conductivity [5]. Three primary techniques have been employed: fins within the PCM system, the nano-enhanced PCM system, and the metal foam PCM (MFPCM) system (Figure 3). These techniques are described in the following subsections.
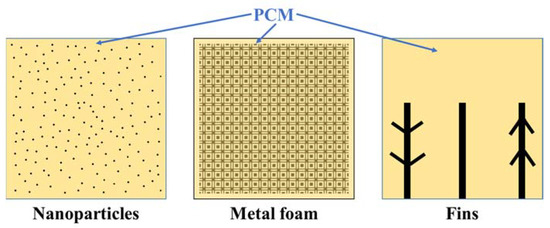
Figure 3.
Example of enhancement added to PCM.
2.1. Finned PCM Systems
In heat transfer, fins are defined as surfaces extending from objects to increase the heat transfer rate. It happens with the increase in surface area. This subsection reviews recent studies exploring fins’ use within latent heat storage units. An example of this usage is shown in Figure 3 (right).
Kuboth et al. [7] developed a simulation model to examine the effect of different fin distributions on the performance of shell-and-tube type latent thermal storage units at discharge. The heat exchanger pipe fin density was increased toward the pipe outlet. The concentration of fins was implemented linearly, exponentially, or suddenly. The PCM for this application had a melting temperature ranging from 40 to 44 °C. The average storage performance at total discharge only increased by three percent with the best allocation compared to an equidistant arrangement. Luo and Liao [8] introduced dendritic fins to improve heat exchangers' solid–liquid phase change. The phase change temperature ranged from 42 to 44 °C. The results indicated that the dendritic fin enhanced the solid–liquid phase change in the heat exchanger for latent thermal storage. The presence of a dendritic fin led to the formation of multiple independent PCM zones. Zarei et al. [9] numerically studied the effects of copper fins’ presence, configuration, and dimensions on a triplex tube containing PCM (melting temperature around 82 °C). Mixed fin configuration produced the highest storage when the fins were 28 and 1 mm long and wide, respectively. Liu et al. [10] investigated the performance of a PCM mesh-finned heat sink for the thermal management of thyristors. They experimented with PCMs with different melting temperatures from 43.5 to 150 °C. The PCM heat sink provided 80 s protection time and 100 s recovery time, demonstrating the excellent potential for this application.
Pakalka et al. [11] compared experimental and numerical natural convection heat transfer coefficients for the PCM melting process in a fin-and-tube copper heat exchanger. The PCM melting temperature ranged between 77 and 82 °C. The results were similar; the coefficients obtained experimentally and numerically were 61 and 68 W/m2K, respectively. Ghalambaz et al. [12] developed and analyzed a three-dimensional model of a twisted-fin array for intensifying the charging response of PCM within a shell-and-tube storage system. They selected a PCM whose melting point is between 29 and 35 °C and compared the proposed configuration against straight fins and no-fins shell-and-tube storage systems. It was found that the energy charging time could be reduced by up to 42%, and the energy storage rate could be enhanced by up to 63%. Sun et al. [13] also analyzed a system with twisted fins but in a triple-tube latent heat energy storage. Torbarina et al. [14] developed a computational model to describe the heat transfer between HTF and PCM in a tank (shell and tube) with straight fins. The PCM used had a melting temperature between 18 and 25 °C. Chen et al. [15] investigated the impact of bifurcated fins on melting and solidification processes and introduced an arc-shaped fin design to improve heat transfer. They utilized a PCM with a phase change temperature from 45 to 51 °C. The findings indicated that applying bifurcated fins could notably decrease the overall entropy generation. Furthermore, the energy storage time and the energy release time were reduced by 52.7 and 51.6%, respectively, with the implementation of concentric arc-shaped fins.
Privitera et al. [16] compared three axial heat conduction structures changed only for the fin shape (rectangular, trapezoidal, and fractal), using the commercial package ANSYS FLUENT for the use of energy storage systems. They used sodium nitrate (NaNO3) as PCM. The fractal fin exhibited the highest discharging power (1136.6 W) against 950.8 and 979.4 W for rectangular and trapezoidal, respectively. Yu et al. [17] investigated the melting performance of a shell-and-tube thermal energy storage unit containing PCM with a rectangular fin configuration. The melting temperature of the PCM was between 48 and 50 °C. Their observations showed that the melting process was initially dominated by heat conduction and then by natural convection. They found that increasing the inlet temperature and reducing the flow rate of the HTF can enhance the energy efficiency of the PCM unit. Liu et al. [18] compared three PCM systems (no fins, circular fin, and spiral fin) for passive thermal management in cylindrical lithium–ion batteries. The utilized PCM had a melting point between 38 and 43 °C and a latent heat of 165 kJ/kg. The best performance was obtained with six turns of 6 mm width-spiral fins. Xu et al. [19] studied the melting rate of PCMs under different structural parameters (length, width, and position) of longitudinal rectangular fins in a horizontal shell-and-tube latent heat storage unit. The PCM used in their experiments had a melting temperature between 47.5 and 48.5 °C.
Rawat et al. [20] numerically analyzed the melting process of PCM in a rectangular enclosure using flanged fins to enhance melting. A double fin arrangement with upper and lower fins was employed, and various cases based on fin length ratio and material were examined with no-fin PCM enclosure. The result showed a high PCM melting rate for a low ratio and high thermal conductivity. Moreover, Cu fins outperformed other cases. The optimization concluded that a single flanged fin placed at the lower end of the container with a ratio of 0.55 has the minimum time (2338 s) and cost (0.229 USD/W). Firstly, Zhang et al. [9] compared and analyzed the thermal performances of nine new fins and the conventional straight fin. They secondly investigated the effects of transverse fin coverage area, number, arc length, thickness, and arc length of inner and outer arc fins on thermal performance, considering the impact of different heat transfer coefficients. The optimal fins kept the average cell temperature within 318.15 K, improved the heat transfer capability by 14.98%, extended the operating time by 131.5%, and reduced the system weight by 10.28%.
2.2. Nano-Enhanced PCM
Nanoparticles have a large surface-to-volume ratio. This high ratio makes them have specific properties. Nanoparticles can have a thermal conductivity of up to 5300 W/m.K depending on the material [21]. Adding nanoparticles to PCM produces a composite (NPCM) with affected thermophysical properties (Figure 3, left). The deposition of nanoparticles is crucial to fight against because if many nanoparticles stick together, the NPCM loses efficiency.
Prabakaran et al. [22] experimentally investigated the solidification behavior of functionalized graphene-based phase change nanocomposites inside a sphere (thermal transport and rheological characteristics). They obtained a maximum thermal conductivity enhancement of approximately 102 and 46%, with 0.5 vol% in the solid and liquid states, respectively. The rheological measurements showed that including nanoparticles led to the transition to non-Newtonian behavior. Pasupathi et al. [23] experimentally studied the influence of hybrid nanoparticles containing silicon dioxide (SiO2) and cerium dioxide (CeO2) nanoparticles on thermo-physical characteristics of the paraffin-based PCM. They examined the synthesized samples under different instruments. The melting and solidification point was 63.74 °C. Mainly, the thermal conductivity of the paraffin was enriched up to 115.49% and 165.56%. Khan and Khan [24] integrated extended fins and graphene nanoparticles into the PCM to enhance thermal performance. The PCM used in their study had a melting temperature range from 41 to 44 °C, and they used different volume percentages of graphene nanoparticles (1, 3, and 5 vol%). The results showed that their proposed configuration could retrieve 11.15 MJ of thermal enthalpy in 1.08 h, significantly faster than the conventional shell-and-tube without fins. It takes 44.5 h to recover the same amount of thermal enthalpy. Mohaghegh et al. [25] studied the heat transfer of water jet impingement on a hot plate enhanced with a nano-encapsulated PCM slurry. The slurry contained polystyrene shells with n-octadecane paraffin wax as the core and aluminum oxide nanoparticles. They found that the maximum cooling performance was achieved with a 15% concentration of nano-encapsulated PCM. The study was focused on the laminar flow regime.
Zahid et al. [26] conducted an experimental study on the thermal behavior of a copper foam-based heat sink integrated with various concentrations (0.15, 0.20, and 0.25 wt%) of alumina nanoparticles and PCM (RT-54HC). The study showed a maximum reduction of 36.95% in the base temperature and a maximum improvement of 288% in the working time. The authors suggested the use of NPCMs in electronic cooling systems. John et al. [27] studied a battery thermal management system based on organic PCM (stearic acid) and copper oxide nanoparticles. They optimized the system for different PCM thicknesses of 2–12 mm and additive percentages of 0–6% of nanoparticles. The results showed that stearic acid-based PCM effectively controls cell temperature. The lowest cell temperature attained was found to be 311.93 K. Hayat et al. [28] incorporated multi-walled carbon nanotubes, graphene nano-platelets, and titanium oxide-based single and novel hybrid nano additives into paraffin to find the optimal composite which could not only enhance the thermal conductivity but also limit the latent heat. The results showed that the highest thermal conductivity value was observed at 1.0 wt% of graphene and multi-walled nanotube hybrid particles based PCM with a maximum enhancement of 170% at 25 °C. Moreover, titanium oxide nanoparticles showed the minimum reduction in the latent heat. They mentioned that nanofillers have the potential to be employed in thermal energy storage applications.
2.3. Metal Foam PCM
The enhancement of heat transfer by pouring PCM inside a metal foam structure is induced by conduction and convection. Metal foam assists the conduction mechanism, suppresses convective heat transfer and its porous produces resistance to the free movement of liquid PCM. Higher porosity and lower pore density of MF can help mitigate the suppression of natural convection [29]. The way PCM fills the empty spaces inside metal foam is presented in Figure 3 (center).
Chen et al. [30] numerically investigated the energy transport inside a PCM-based thermal energy storage system using metal foam as an enhancement technique. The PCM changed its phase at 58 °C, approximately. The results showed that the overall performance improved by inserting metal foam in both HTF and PCM sides, and the time needed for the entire process decreased by 84.9% compared to the case of pure PCM. Mabrouk et al. [31] numerically investigated the two-dimensional laminar flow and the heat transfer in an open-ended rectangular porous channel (metal foam), including a PCM under forced convection. They found the foam pore effect on the phase change process under unsteady forced convection in a PCM-saturated porous channel. Ghalambaz et al. [32] studied the melting flow and heat transfer of copper-oxide coconut oil in thermal energy storage filled with a nonlinear copper metal foam. They investigated the effect of average porosity; porosity distribution; pore size density; the enclosure’s inclination angle, and nanoparticles’ concentration on the isotherms, melting maps, and melting rate. The coconut oil changed its phase at 24 °C. The results showed that the natural convection flows were weak in the metal foam, and the variation in porosity from 0.825 to 0.9 changes the melting time by about 116%. Ghalambaz et al. [33] investigated a thermal energy storage system consisting of a cylinder (filled with an inhomogeneous porous medium) with a longitudinal inner tube containing the HTF. They also used coconut oil loaded with copper nanoparticles. Their results showed that increasing nanoparticle volume fraction decreased the charging time.
Falcone et al. [34] showed the benefits that a 95% porous copper metal foam could bring to a PCM-based thermal storage system by simply loading it due to the increase in the effective thermal conductivity of the medium. They used a PCM in which phase change temperature ranged from 29 to 36 °C. They noticed that metal foam produced a more even temperature within the PCM. On the other hand, the melting time was reduced. Liu et al. [35] developed a heat storage system with metal foam and three structures (positive, negative, and no gradient) as an enhancer to save and deploy waste heat. The melting temperature of the PCM was between 46 and 55 °C. The total phase transition time was 12,470 s, 13,500 s, and 17,930 s in the cases of positive, no, and negative gradients, respectively. The initial investment increased by 76.09%, compared to not adding metal foam, but it was cost-effective in the long run of thermal cycles. Hassan et al. [36] used NPCM with copper foam to cool electronic devices. They combined PT-58 PCM with graphene nanoplatelets and magnesium oxide (MgO) nanoparticles. The research concluded that incorporating copper foam in the NPCM can effectively decrease the heat sink base temperature and provide the best cooling performance at low and high heating loads.
Chen et al. [37] experimentally and numerically studied the influence mechanism of the physical parameters of PCMs and structural parameters of metal foam on the melting of composite PCMs. The result showed that the porosity, the material of the metal foam, and the thickness of the metal foam were the main factors affecting the melting rate and heat transfer strength of PCMs. In contrast, the effect of pore density can be ignored. They analyzed and obtained three general correlations about the liquid fraction; Nusselt number with Fourier number, Stefan number, and Rayleigh number; and dimensionless structural parameters of metal foams. Sreenath and Chanda [38] studied the effect of ambient conditions and choice of PCM on the thermal performance of a metal foam composite heat sink. The PCMs considered were paraffin wax, docosane, and eicosane. The melting temperatures were 44.5–60.1 °C, 40.3–44.5 °C, and 36.3–38.1 °C, respectively. They found that the temperature uniformity within the heat sink cavity improved significantly in the presence of metal foam. However, due to the limit in thermal conductivity enhancement that can be achieved by introducing high thermally conductive foam material into the PCM material, similar behavior concerning the enhancement of temperature uniformity and attainment of maximum base temperature was observed with aluminum and copper foam PCM composite heat sinks.
References
- Ban, K.M. Sustainable Development Goals. News Surv. 2016, 37, 18–19.
- Reddy, K.G.; Deepak, T.G.; Anjusree, G.S.; Thomas, S.; Vadukumpully, S.; Subramanian, K.R.V.; Nair, S.V.; Nair, A.S. On Global Energy Scenario, Dye-Sensitized Solar Cells and the Promise of Nanotechnology. Phys. Chem. Chem. Phys. 2014, 16, 6838–6858.
- Belyakov, N. Chapter Twenty-Three—Sustainable Electricity Management beyond Generation. In Sustainable Power Generation; Belyakov, N., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 539–563. ISBN 978-0-12-817012-0.
- Hussain, F.; Rahman, M.Z.; Sivasengaran, A.N.; Hasanuzzaman, M. Chapter 6—Energy Storage Technologies. In Energy for Sustainable Development; Hasanuzzaman, M.D., Rahim, N.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 125–165. ISBN 978-0-12-814645-3.
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of Solid-Liquid Phase Change Materials and Their Encapsulation Technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391.
- Javadi, F.S.; Metselaar, H.S.C.; Ganesan, P. Performance Improvement of Solar Thermal Systems Integrated with Phase Change Materials (PCM), a Review. Sol. Energy 2020, 206, 330–352.
- Kuboth, S.; König-Haagen, A.; Brüggemann, D. Numerical Analysis of Shell-and-Tube Type Latent Thermal Energy Storage Performance with Different Arrangements of Circular Fins. Energies 2017, 10, 274.
- Luo, X.; Liao, S. Numerical Study on Melting Heat Transfer in Dendritic Heat Exchangers. Energies 2018, 11, 2504.
- Zarei, M.J.; Bazai, H.; Sharifpur, M.; Mahian, O.; Shabani, B. The Effects of Fin Parameters on the Solidification of PCMs in a Fin-Enhanced Thermal Energy Storage System. Energies 2020, 13, 198.
- Liu, J.; Yu, S.; Yang, S.; Zhang, Y.; Fan, X.; Gao, B. Numerical Studies on the Performance of the PCM Mesh-Finned Heat Sink Base on Thermal-Flow Multiphysics Coupling Simulation. Energies 2020, 13, 4658.
- Pakalka, S.; Valančius, K.; Streckienė, G. Experimental and Theoretical Investigation of the Natural Convection Heat Transfer Coefficient in Phase Change Material (PCM) Based Fin-and-Tube Heat Exchanger. Energies 2021, 14, 716.
- Ghalambaz, M.; Mohammed, H.I.; Mahdi, J.M.; Eisapour, A.H.; Younis, O.; Ghosh, A.; Talebizadehsardari, P.; Yaïci, W. Intensifying the Charging Response of a Phase-Change Material with Twisted Fin Arrays in a Shell-And-Tube Storage System. Energies 2021, 14, 1619.
- Sun, X.; Mahdi, J.M.; Mohammed, H.I.; Majdi, H.S.; Zixiong, W.; Talebizadehsardari, P. Solidification Enhancement in a Triple-Tube Latent Heat Energy Storage System Using Twisted Fins. Energies 2021, 14, 7179.
- Torbarina, F.; Lenic, K.; Trp, A. Computational Model of Shell and Finned Tube Latent Thermal Energy Storage Developed as a New TRNSYS Type. Energies 2022, 15, 2434.
- Chen, Q.; Wu, J.; Sun, K.; Zhang, Y. Numerical Study of Heat Transfer Enhancement by Arc-Shaped Fins in a Shell-Tube Thermal Energy Storage Unit. Energies 2022, 15, 7799.
- Privitera, E.; Caponetto, R.; Matera, F.; Vasta, S. Impact of Geometry on a Thermal-Energy Storage Finned Tube during the Discharging Process. Energies 2022, 15, 7950.
- Yu, M.; Sun, X.; Su, W.; Li, D.; Shen, J.; Zhang, X.; Jiang, L. Investigation on the Melting Performance of a Phase Change Material Based on a Shell-and-Tube Thermal Energy Storage Unit with a Rectangular Fin Configuration. Energies 2022, 15, 8200.
- Liu, J.; Ma, Q.; Li, X. Numerical Simulation of the Combination of Novel Spiral Fin and Phase Change Material for Cylindrical Lithium-Ion Batteries in Passive Thermal Management. Energies 2022, 15, 8847.
- Xu, Y.; Yin, H.; He, C.; Wei, Y.; Cui, M.; Zheng, Z.-J. Structure Optimization of Longitudinal Rectangular Fins to Improve the Melting Performance of Phase Change Materials through Genetic Algorithm. Energies 2022, 15, 9610.
- Rawat, P.; Ashwni; Sherwani, A.F. A Numerical Study on the Impact of Fin Length Arrangement and Material on the Melting of PCM in a Rectangular Enclosure. Int. J. Heat Mass Transf. 2023, 205, 123932.
- Yang, L.; Huang, J.; Zhou, F. Thermophysical Properties and Applications of Nano-Enhanced PCMs: An Update Review. Energy Convers. Manag. 2020, 214, 112876.
- Prabakaran, R.; Sidney, S.; Lal, D.M.; Selvam, C.; Harish, S. Solidification of Graphene-Assisted Phase Change Nanocomposites inside a Sphere for Cold Storage Applications. Energies 2019, 12, 3473.
- Pasupathi, M.K.; Alagar, K.; Mm, M.; Aritra, G. Characterization of Hybrid-Nano/Paraffin Organic Phase Change Material for Thermal Energy Storage Applications in Solar Thermal Systems. Energies 2020, 13, 5079.
- Khan, Z.; Khan, Z.A. Performance Evaluation of Coupled Thermal Enhancement through Novel Wire-Wound Fins Design and Graphene Nano-Platelets in Shell-and-Tube Latent Heat Storage System. Energies 2021, 14, 3743.
- Mohaghegh, M.R.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Jet Impingement Cooling Enhanced with Nano-Encapsulated PCM. Energies 2022, 15, 1034.
- Zahid, I.; Farooq, M.; Farhan, M.; Usman, M.; Qamar, A.; Imran, M.; Alqahtani, M.A.; Anwar, S.; Sultan, M.; Javaid, M.Y. Thermal Performance Analysis of Various Heat Sinks Based on Alumina NePCM for Passive Cooling of Electronic Components: An Experimental Study. Energies 2022, 15, 8416.
- John, S.; Sreyas, K.; Mohan, Y.; Thampi, A.D.; Rani, S. Numerical Investigation on the Effect of PCM Thickness and Nano-Additive on the Cooling Performance of Stearic Acid Based Battery Thermal Management System. Mater. Today Proc. 2023, in press.
- Aamer Hayat, M.; Yang, Y.; Li, L.; Bevilacqua, M.; Kang Chen, Y. Preparation and Thermophysical Characterisation Analysis of Potential Nano-Phase Transition Materials for Thermal Energy Storage Applications. J. Mol. Liq. 2023, 376, 121464.
- Aramesh, M.; Shabani, B. Metal Foams Application to Enhance the Thermal Performance of Phase Change Materials: A Review of Experimental Studies to Understand the Mechanisms. J. Energy Storage 2022, 50, 104650.
- Chen, X.; Li, X.; Xia, X.; Sun, C.; Liu, R. Thermal Performance of a PCM-Based Thermal Energy Storage with Metal Foam Enhancement. Energies 2019, 12, 3275.
- Mabrouk, R.; Naji, H.; Dhahri, H.; Younsi, Z. Insight into Foam Pore Effect on Phase Change Process in a Plane Channel under Forced Convection Using the Thermal Lattice Boltzmann Method. Energies 2020, 13, 3979.
- Ghalambaz, M.; Shahabadi, M.; Mehryan, S.A.M.; Sheremet, M.; Younis, O.; Talebizadehsardari, P.; Yaici, W. Latent Heat Thermal Storage of Nano-Enhanced Phase Change Material Filled by Copper Foam with Linear Porosity Variation in Vertical Direction. Energies 2021, 14, 3979.
- Ghalambaz, M.; Mehryan, S.A.M.; Shirivand, H.; Shalbafi, F.; Younis, O.; Inthavong, K.; Ahmadi, G.; Talebizadehsardari, P. Simulation of a Fast-Charging Porous Thermal Energy Storage System Saturated with a Nano-Enhanced Phase Change Material. Energies 2021, 14, 1508.
- Falcone, M.; Rehman, D.; Dongellini, M.; Naldi, C.; Pulvirenti, B.; Morini, G.L. Experimental Investigation on Latent Thermal Energy Storages (LTESs) Based on Pure and Copper-Foam-Loaded PCMs. Energies 2022, 15, 4894.
- Liu, G.; Li, Y.; Wei, P.; Xiao, T.; Meng, X.; Yang, X. Thermo-Economic Assessments on a Heat Storage Tank Filled with Graded Metal Foam. Energies 2022, 15, 7213.
- Hassan, F.; Hussain, A.; Jamil, F.; Arshad, A.; Ali, H.M. Passive Cooling Analysis of an Electronic Chipset Using Nanoparticles and Metal-Foam Composite PCM: An Experimental Study. Energies 2022, 15, 8746.
- Chen, C.; Diao, Y.; Zhao, Y.; Wang, Z.; Liu, Y.; Han, Y.; Zhu, T.; Fang, D.; Li, J. Melting Performance of a Cold Energy Storage Device Filled with Metal Foam–Composite Phase-Change Materials. J. Energy Storage 2023, 60, 106567.
- Sreenath, V.R.; Chanda, S. Thermal Performance Evaluation of PCM-MF Composite Heat Sinks under Varying Ambient Conditions. Int. J. Heat Mass Transf. 2023, 206, 123927.
More
