Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Wenzeng Duan and Version 3 by Conner Chen.
The [2.2]paracyclophane (PCP) ring has attracted extensive attention due to its features of providing not only chirality and electron-donating ability but also steric hindrance, which reduces intermolecular π–π stacking interactions and thereby improves the fluorescence properties of dyes.
- fluorescent dye
- fluorescent probe
- AICPL
1. Introduction
[2.2]Paracyclophane (PCP) is a typical cyclophane that was first synthesized and isolated in 1949 as a pyrolysis product of para-xylene [1]. PCP includes two strongly interacting benzene unit “decks” with a distance of ~3.09 Å, which are connected by two ethylene “bridges” (~2.78 Å) (Figure 1) [2]. The two benzene rings stacked at such close proximity can lead to transannular π–π strain in the aromatic rings and cause deviation from the normal molecular structure. The strain, distorted structure and transannular effects alter the photophysical and optoelectronic properties of PCP scaffolds. Therefore, due to the intriguing structure and the unusual photophysical [3] and optoelectronic properties [4] of PCP scaffolds, they have been successfully used as privileged scaffolds in asymmetric synthesis [5], π-stacked polymers [6], energy materials [7], and organic fluorescent dyes [8] and have aroused wide interest in biological and materials science fields [9].
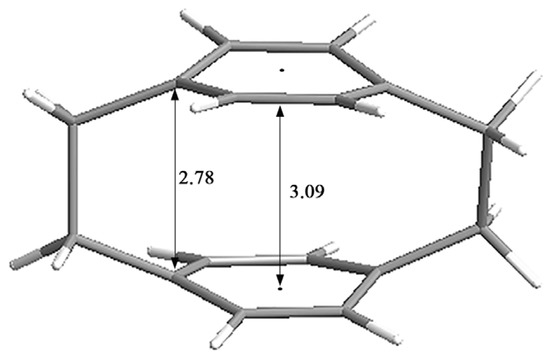
Figure 1.
Strain and structural parameters in ångstroms [Å] of PCP.
Conventional organic fluorescent dyes include cyanines [10], BODIPYs (4,4-difluoro-4-bora-3a,4a-diazas-indacene) [11], rhodamine analogues [12], squaraines [13], and porphyrins [14]. BODIPY dyes have a large π-conjugated skeleton and exhibit excellent photophysical properties in dilute solution. However, when in high concentration in solution or a state of aggregation, BODIPY dyes tend to adopt a face-to-face stacking mode (H-aggregation) and usually display an aggregation-caused emission quenching (ACQ) effect, which limits their application. Fortunately, the ACQ effect can be effectively overcome using an aggregation-induced emission (AIE) strategy, which was introduced by Tang et al. and has since been widely used [15]. Many AIE skeletons, such as 1,1,2,3,4,5-hexaphenylsilone and tetraphenylethylene, have been developed and applied in organic fluorescent dyes. These skeletons have twisted structures and can effectively inhibit the intermolecular π–π interactions occurring in the aggregation state. Taking advantage of PCP scaffolds, the introduction of a PCP group to BODIPY or BODIPY analogues can also result in elimination of the strong π–π interactions between the intermolecular indacene planes and achieve an AIE effect. Therefore, the introduction of the PCP skeleton to organic fluorescent dyes improves the photophysical properties of fluorescent dyes and has received increasing attention in recent decades.
Circularly polarized luminescence (CPL) has many potential applications in chirality sensing [16], optical displays [17][18][19][17,18,19], and chiroptical materials [20][21][20,21]. The two key factors to satisfy the requirements of the above applications during the development of CPL materials are high fluorescence quantum yield (Φf) and high luminescent dissymmetry factors (|glum|) [22]. In recent years, CPL-active small organic molecules (CPL-SOMs) have received wide attention due to their easy derivatization, excellent processability, tunable wavelengths, high fluorescence quantum yields, and low toxicity [23][24][25][26][27][23,24,25,26,27]. However, many CPL-SOMs suffer from the disadvantages of small |glum| values and aggregation-caused quenching (ACQ). An AIE strategy is also effective for achieving higher Φf and larger |glum| values, and has been applied to overcome ACQ in CPL-SOMs [28]. It is well known that AIE-active SOMs display no or weak fluorescence in dilute solution but become highly emissive as aggregates or in a solid state, which may increase Φf and amplify the |glum| values.
2. Application of PCP Skeleton in Modifying Dyes
It is well known that many reported organic fluorescent dyes suffer from small Stokes shifts, which affects their fluorescence emission efficiency. Therefore, it is necessary to develop a fluorescent dye with large Stokes shifts, superior optical properties, and good stability [29][31]. Due to the special electronic structure, [2.2]paracyclophanyl fluorescent dyes exhibit intrinsic fluorescence and can be applied to access new fluorescent dyes [30][32]. In 2019, Delcourt’s group reported a series of [2.2]paracyclophane-fused coumarin systems (Scheme 1) [31][33], which are compact heteroaromatic PCP dyes with unique three-dimensional (3D) structures. The introduction of the PCP skeleton allows the synthesis of 3D coumarin systems and improvement of the photophysical properties through large Stokes shifts and red-shifted absorption and emission bands. Furthermore, when planar chiral 4-formyl [2.2]paracyclophane was introduced to these [2.2]paracyclophane-fused coumarins, the resulting product exhibited promising chiroptical properties with |glum| up to 5 × 10−3.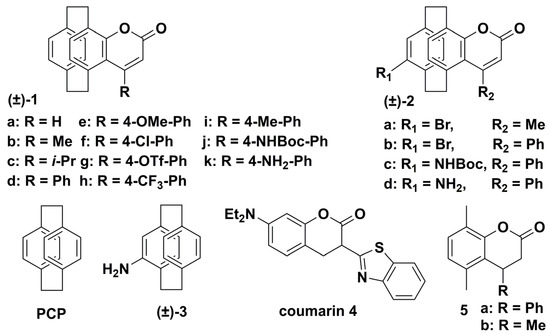
Scheme 1.
Chemical structures of PCP and PCP-related emitters (1−5).
Table 1.
Structures and spectroscopic properties of PCP-related fluorescent dyes 1−14.
| Entry | Emitter | Φf | λabs (nm) | λem in Solution (nm) | λem in Other States (nm) | References |
|---|---|---|---|---|---|---|
| 1 | PCP | - | 286, 302 | 356 | - | [31][33] |
| 2 | 1a | - | 304, 320 | 445 | - | [31][33] |
| 3 | 1d | - | 265, 300, 330 | 460 | - | [31][33] |
| 4 | 1h | - | 270, 335 | 475 | - | [31][33] |
| 5 | 1k | - | 330 | 470 | - | [31][33] |
| 6 | 2b | - | 307, 325 | 450 | - | [31][33] |
| 7 | 3 | - | 274, 324 | 386 | - | [31][33] |
| 8 | 4 | - | 455 | 498 | - | [31][33] |
| 9 | 5a | - | 294 | 418 | - | [31][33] |
| 10 | 6d | 0.87 | 589 | 619 | 636 ± 4 in amorphous deposits |
[32][34] |
| 11 | 7 | 0.95 | 543 | 556 | 636 ± 4 in amorphous deposits |
[32][34] |
| 12 | 8 | 0.064 | 722 | 795 | 1010 in aggregated state | [33][35] |
| 13 | 9 | - | 700 | 750 | - | [33][35] |
| 14 | 10a | - | - | 580 | 600 in the addition of Hg2+ | [34][36] |
| 15 | 11c | 0.95 | 382 | 490 | 550 in solid state | [35][37] |
| 16 | 12a | 0.39 | 325, 407 | 543 | 534 in aggregated state | [36][38] |
| 17 | 13 | 0.07 | 341, 423 | 534 | 531 in aggregated state | [37][29] |
| 18 | 14a | 0.56 | 410, 470 | 641 | 686 in solid state | [38][30] |
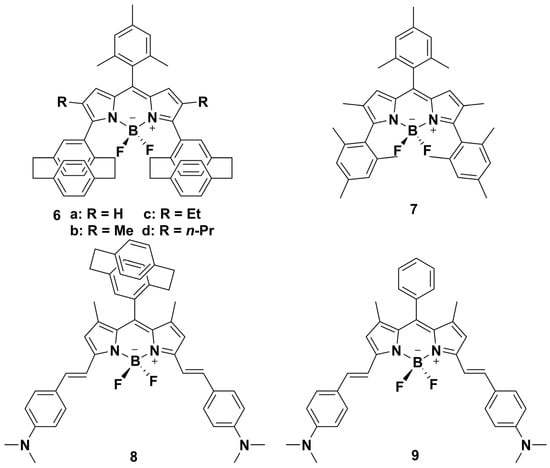
Scheme 2.
Chemical structures of PCP-BODIPY and Ph-BODIPY (6−9).
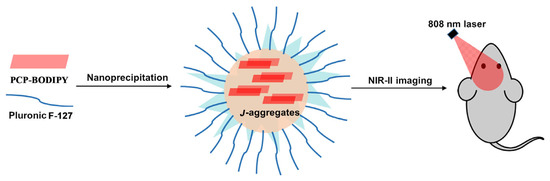
Figure 2.
The NIR-II imaging of PCP-BODIPY in nanoparticles (NPs).
