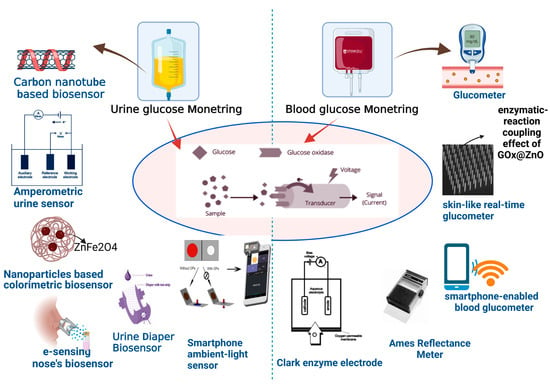
1. Urine Glucose Monitoring
Elevated glucose levels in urine are considered dangerous since they suggest the progression of diabetes. A positive urine result shows glucose levels in the system are greater than 50–100 mg/dL (2.78–5.55 mM)
[1][21]. In 1996, the first Japanese urine glucose meter significantly monitored urine glucose levels from 0 to 500 mg/dL
[2][3][22,23]. In 1999, the TOTO Corporation released a urine glucose monitor device that was incorporated into toilet seats. The built-in meter was used to monitor diluted urine samples (
Figure 12).
Figure 12.
Diagnosis of glucose level in urine and blood with help of modern biosensor tools.
Carbon nanotubes (CNTs) have also been investigated to detect glucose in urine. Dissolved CNTs in biopolymer chitosan (CS) aqueous solutions allow urine glucose measurement without interference (with a detection limit of 3 M)
[4][5][3,24]. Kimura Group’s UG-201 is an extremely sensitive urine glucose meter that uses an Amperometric glucose sensor. It has outstanding performance characteristics, such as a broad measuring range of 0–2000 mg/dL, a rapid reaction time of 6 s, and resistance to interference from interferents, such as ascorbic acid/acetaminophen. There was an excellent linear association between the developed urine glucose meter and a commercially available urine glucose analyzer for clinical application. The developed urine glucose meter was used to monitor post-meal blood glucose levels by assessing the urine glucose of actual people. The new portable meter was created to use locations other than the house or office
[6][4].
According to research published in 2016, ZnFe
2O
4 magnetic nanoparticles (MNPs) with in-built peroxidase-like activity could be used as a colorimetric biosensor for detecting glucose in urine. For glucose detection utilizing glucose oxidase (GOx), ZnFe
2O4 MNPs are affordable, highly sensitive, and selective, with a linear range of 1.25 × 10
−6 to 1.875 × 10
−5 mol/L (1.25–18 µM) and a detection limit of 3.0 × 10
−7 mol/L (0.3 µM)
[7][25]. Using an electronic nose, Siyang et al. suggested a new approach for detecting diabetes based on a direct assessment of urine odor (e-nose). The e-sensing nose’s parts were made up of eight commercial chemical gas sensors. Cluster analysis (CA) and principal components analysis (PCA) approaches were used to study the data. The method successfully determined glucose levels in urine
[8][6].
Wang et al. created a smartphone ambient-light sensor and a label-free colorimetric test for determining urine glucose (ALS). Quantitative H
2O
2 was applied to samples to determine the deepest color using the horseradish peroxidase hydrogen peroxide 3,3′,5,5′-tetramethylbenzidine (HRP-H
2O
2-TMB) system, a smartphone ambient-light sensor was attached to assess transmitted light illuminance, and color variations were used to compute urine glucose concentration
[9][26].
Zhang et al. developed a flexible self-powered biosensor device connected to a diaper to detect urine composition. The device was powered by an enzyme biofuel cell (EBFC), which generated energy using glucose from urine as fuel. The biosensor system, which consisted of EBFC, a power management system, and a light-emitting diode, was a self-powered sensor that could detect glucose levels in diabetes patients’ urine without the use of external power
[10][27]. Lee et al. developed a paper sensor composed of polyaniline nanoparticles (PAni-NPs)
[11][28] and noninvasive, intuitive, and highly selective red blood cell membranes (RBCMs)
[12][29]. The glucose transporter-1 protein in RBCM (coated on PAni-NP-adsorbed paper) functions as an intelligent filter, transferring glucose while rejecting other interfering molecules. The RBCM-coated PAni-NP-based paper sensor was about 85 percent more selective than uncoated paper sensors. The paper sensor could detect 0.54 mM and could detect urine glucose concentrations between 0 mg/mL and 10 mg/mL (0–56 mM). The creation of a highly specific colorimetric urine glucose monitoring device is made possible by this paper sensor
[13][30].
2. Blood Glucose Monitoring
Due to the enormous number of disease-related markers and the less intrusive collection process, blood has been the most extensively utilized sample type. As a result, it becomes a useful supply of biomarkers for biosensors that may be carried around
[14][31]. Monitoring fasting plasma glucose levels is inadequate for achieving effective post-meal glucose management
[15][32]. Recent research suggests that lowering post-meal plasma glucose is critical for achieving reduced hemoglobin A1c (HbA1c), a marker of diabetes progression
[15][32]. The self-monitoring of blood glucose is extensively proven as an efficient strategy for controlling diabetic blood glucose levels, and much work has gone into designing blood glucose sensors
[16][33].
2.1. Glucometer
As previously indicated, commercial glucose sensors rely on puncture tests that should be performed up to seven times daily. Abbott Diabetes Care Ltd. Omron Healthcare, Inc. and Roche Diagnostics Ltd. are among the companies that sell finger-pricking sensors for personal usage (Accu-Chek). Glucometers, a well-known and advanced technology that gives accurate results quickly and with high sensitivity, are the most commonly used conventional sensors for managing glucose
[17][34]. The blood glucometer was initially presented in 1970 as a semiquantitative and visual instrument for estimating glucose levels by matching a pad’s glucose-specific response to a printed color scheme
[18][35]. Even though the initial blood glucometer needed numerous stages, a considerable blood volume, and precise timing, they could help people with diabetes regulate their blood glucose levels. The redox potentials of the two enzymes (GOx at −48 mV vs. SHE at pH 7.2 and GDH at 10.5 mV vs. SHE at pH 7.0), stability, turnover rates, and glucose affinity and selectivity are all different
[19][36]. GOx has a more robust selectivity for glucose than GDH and can survive more significant pH, ionic strength, and temperature fluctuations. GOx, on the other hand, catalyzes glucose oxidation at a rate of 5000 s
−1, compared with GDH’s rate of 11,800
[20][37]. After glucose is oxidized, a mediator, such as ferrocene derivatives, hexacyanoferrate, or quinones, carries the enzyme signal to the working electrode.
Despite the commercial success of contemporary mediator-based BGM systems, it has been shown that some medications, metabolites, and other blood components may interact with these glucose sensors
[21][38]. Blood glucometer devices available today are adequate for monitoring glucose levels in diabetics; however, 95% of the measured glucose readings must be within 15 mg/dL (0.83 mM) of the reference measurement at glucose concentrations of 100 mg/dL (5.6 mM) or within 15% at glucose concentrations of 100 mg/dL (5.6 mM), according to the ISO standard
[22][39]. Given the variety of variables that might affect glucose measurements, such as hematocrit, temperature, altitude, and human error
[23][40], one of the few commonly used in vitro diagnostic devices that have been upgraded with cellular capabilities and mHealth apps is the blood glucose meter.
2.2. Colorimetric Strip
Xue et al. developed a glucometer that is flexible, self-powered, and skin-like to monitor blood glucose levels in the body in real-time for the prevention and treatment of diabetes. The operative mechanism is based on the coupling effect between piezoelectricity and enzyme reactions in arrays of GOx@ZnO nanowires. Under strain, the device converts mechanical energy into piezoelectric impulses. Blood glucose levels affect this process. The piezoelectric voltage generates electricity and biosensing signals. The implanted device can check blood glucose levels live
[24][19].
As a non-glucose quantitative portable detection system, Li et al. proposed a unique design that combines a classic blood glucose monitoring device using a lateral flow strip and a commercially available smartphone (iBGStar
® Blood Glucose Monitoring System). An oxidative DNA impairment biomarker, 8-hydroxy-2′-deoxyguanosine, is used to demonstrate the notion (8-OHdG). The device’s fundamental design is based on a gold nanoparticles (AuNPs)-based competitive immunoassay, as described in the colorimetric visual detection platform. Visual detection, on the contrary, provides only qualitative and somewhat quantitative information. Quantitative analysis is made possible by switching from target detection to enzyme invertase detection with this method
[25][41].
2.3. Ames Reflectance Meter
In 1970, Anton H. Clemens created the first blood glucose meter, the Ames Reflectance Meter (ARM), which used reflected light from a Dextrostix strip to determine a person’s blood sugar level
[26][42]. A significant drop of blood (about 50–100 L) was put into the reagent pad, which was gently rinsed away after one minute, and the pad color was visually analyzed against a color chart to provide a semiquantitative blood glucose measurement. Reflocheck, a tiny portable reflectance meter that used Reflotest strips that were cleaned with a cotton ball and included a barcode for calibration, was introduced by Boehringer Mannheim (BM) in 1982. The findings of a diabetic screening trial in general practice revealed cost savings, and the evaluations showed remarkable correlation and precision
[27][43].
3. Glucose Biosensors
3.1. The Clark Enzyme Electrode
The most popular interstitial fluid (ISF) analysis technique for clinical continuous glucose monitoring remains implanted enzyme-electrode sensors
[28][44]. Because the active working electrode (WE) is commonly made on the cylindrical-end face, this type of implantable sensor requires a tiny diameter such that the WE area is very small. The sensor’s sensitivity is limited due to the narrow WE area. As a result, typical enzyme–electrode sensors struggle to detect hypoglycemia, which is a severe disease
[29][45]. To reduce signal drift and enable hypoglycemia diagnosis, a new cylindrical, flexible enzyme–electrode sensor with a larger WE surface is presented for implanted continuous glucose monitoring. A cylindrical substrate was used because it retains the most surface area for a given volume compared with other geometries, and a larger cylindrical surface creates a larger WE surface. By passing the diameter restriction imposed by conventional pin-like enzyme–electrode sensors on a cylindrical surface, the WE may be formed not only along the radius but also along the axis of the cylindrical substrate
[29][45].
Attaching the appropriate oxidase enzyme to the tip of a Clark-type oxygen microelectrode resulted in microsensors for glucose. The enzyme is immobilized in a polyacrylamide matrix on the electrode tip before being covered with a polyurethane membrane. The quantity of oxygen used by the electrode, and hence the biosensor’s output, is controlled by the analyte concentration in the sample. These micro biosensors had tip sizes ranging from 15 to 40 m, reaction durations ranging from 0.5 to 5 s, and could detect as little as 2 m of the analyte. These microsensors proved a versatile instrument for monitoring particular analytes in unstirred settings, with a spatial resolution of 100 µm or less and speedy response times
[30][46].
3.2. Yellow Springs Instrument
The Yellow Springs Instrument (YSI) 2300 STAT PLUS Glucose and l-Lactate Analyzer (YSI 2300) is the most extensively used comparator instrument for manufacturers to calibrate batches of glucose test strips during manufacturing for self-management blood glucose devices. This gadget has also been frequently used in investigations to show that continuous and exploratory noninvasive glucose monitors are accurate
[31][47].
3.3. Mediated Biosensors
The ultimate objective of glucose monitoring is noninvasive glucose sensing, and the following are the fundamental techniques being investigated for glucose sensor development
[32][48]. Despite the relatively simple use, rapidity, and low danger of infection associated with infrared spectroscopy, this technology is vulnerable to frequent calibrations, poor selectivity, limited sensitivity, and miniaturization challenges. The lack of a correlation between ejected fluids and blood glucose concentrations is one of the concerns, with direct glucose monitoring using void physiological fluids. Changes in glucose concentrations in the fluids caused by exercise and nutrition can also lead to erroneous readings
[33][49]. The ambition to construct an artificial pancreas motivates researchers to keep working on biosensors. However, the shortcomings of in vivo biosensors before creating such an insulin-modulating gadget must be addressed
[34][50].
A variety of glucose sensors are available, most of which are intrusive to the patient. It has been shown that fiber optic sensors provide advantages over conventional sensors and have great application potential, notably in the healthcare sector
[35][51]. Compared to other sensors, they are smaller, more manageable, and mainly noninvasive, resulting in a reduced risk of infection, as well as being accurate, well-correlated, and inexpensive. Different devices based on other approaches, such as optical polarimetry
[36][52] and electrode detection
[37][53], and fluorescence with devices created for glucose management beneath the skin, such as the Senseonics Eversense, have been developed alongside this technology.
In fluorescent glucose testing, the intensity or duration of signal decay may be assessed. The fluorescence lifetime is different for each analyte, which may be evaluated in dispersion media
[38][54] and which aids in distinguishing between substances. Water absorbs less radiation in spectroscopy; hence, light may permeate through the epidermis stratum corneum to generate a higher blood concentration regardless of skin color, making it the material of choice for building noninvasive glucose sensors
[39][55]. Electromagnetism-based technology, on the other hand, monitors glucose using voltage or current based on magnetic coupling
[40][56]. This process depends on the current or voltage connection between the output and input, which is directly proportional to the glucose concentration.