You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Carlos Eduardo Fonseca-Alves.
Hemangiosarcoma is a mesenchymal neoplasm that originates in the endothelial cells of blood vessels. According to the location of origin, they can be classified as non-visceral and visceral types. Hemangiosarcoma can be very aggressive and metastasize to distant organs.
- endothelial
- angiosarcoma
- cutaneous
1. Canine Hemangiosarcoma and Its Different Forms
Hemangiosarcoma (HSA) is a mesenchymal neoplasm originating from the endothelial cells of the blood vessels. It is also called a malignant hemangioendothelioma or angiosarcoma, or Visceral Vascular Tumor, which includes hemangiomas and hemangiosarcomas. The benign counterpart of hemangiosarcoma is hemangioma [1].
Hemangiosarcomas are categorized into two types according to their location or origin: non-visceral hemangiosarcomas and visceral hemangiosarcomas. Non-visceral hemangiosarcomas can affect the skin, subcutaneous tissues, and muscle tissues (Figure 1). In contrast, visceral hemangiosarcomas can affect the spleen, liver, heart, lungs, kidneys, oral cavity, bones, bladder, uterus, tongue, and retroperitoneum [1].
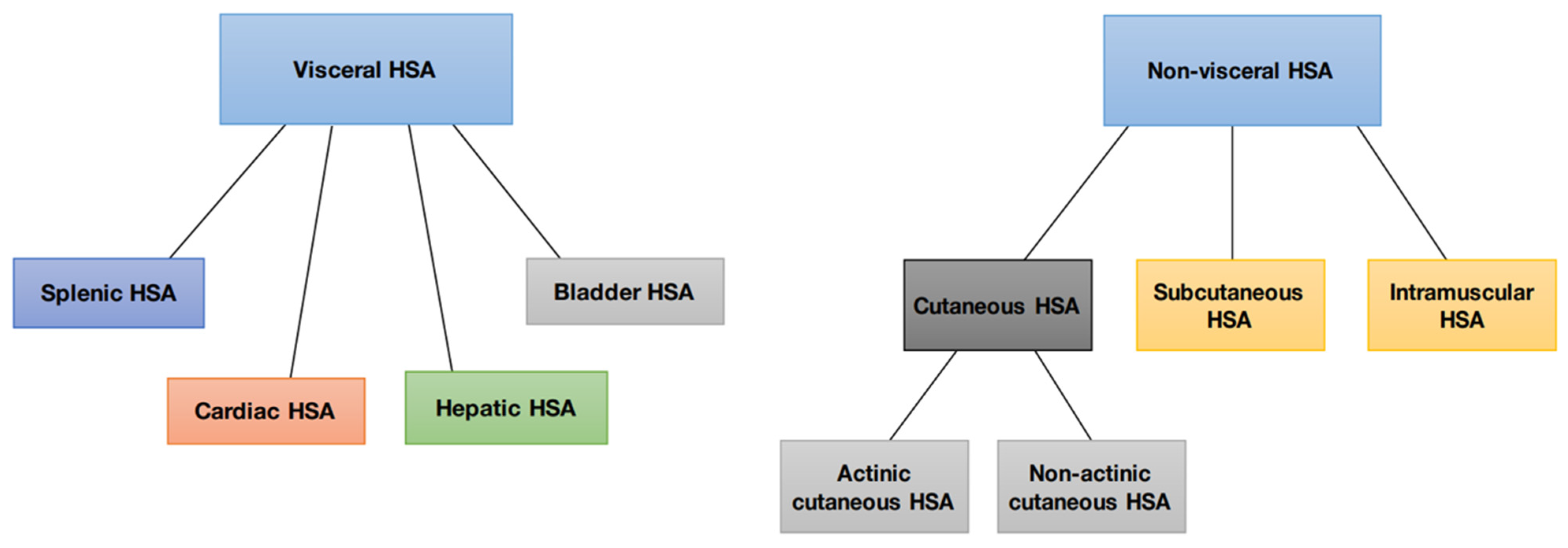
Figure 1.
Schematic representation of the division of canine hemangiosarcoma into visceral and non-visceral.
2. Etiology and Epidemiology
2.1. Actinic and Non-Actinic Cutaneous HSA
2.1. Actinic and Non-Actinic Cutaneous Hemangiosarcoma
Among domestic species, dogs are most affected by cutaneous HSA. Cutaneous HSA represents approximately 14% of all HSA diagnosed in this species and less than 5% of dermal tumors, according to North American studies [3,4,5][2][3][4]. However, Brazilian epidemiological data demonstrate a higher prevalence, which may represent 27 to 80% of all canine HSAs [6,7][5][6] and 13.9% of all skin neoplasms diagnosed in this species [8][7]. This difference is mainly associated with the substantial incidence of solar radiation in countries with tropical climates [9][8]. According to data from the National Institute for Space Research [10][9], Brazil has a high level of ultraviolet radiation, exceeding a scale of 6.0 during most of the year, a factor directly related to the etiology of cutaneous actinic HSA. It is important to note, however, that these epidemiological studies did not differentiate between the actinic and non-actinic subtypes.
Cutaneous HSA most commonly affects middle-aged to elderly dogs (between 8 and 15 years old), with no gender predisposition for either the actinic or non-actinic forms [2,11,12][10][11][12]. Dogs of breeds with lightly pigmented and glabrous skin are at higher risk of developing cutaneous HSA, mainly in its actinic form. The most reported breeds include Pitbull, Whippet, Greyhound, Boxer, Beagle, and Dalmatian [2,3,11,13][2][10][11][13].
The higher prevalence of cutaneous HSA in these animals is related to lower protection from solar radiation, as low skin pigmentation and hair coverage lead to greater sun exposure. Actinic changes, such as solar dermatosis, are frequent in these patients, confirming the influence of solar radiation on the development of this neoplasm [11,13,14][11][13][14]. Such changes occur progressively, and often, affected dogs initially develop hemangiomas that can progress to HSA upon undergoing malignant transformation [15].
Acute exposure to ultraviolet B (UVB) radiation causes skin inflammation and oxidative stress, and long-term exposure to UVB radiation can lead to carcinogenesis. Evidence has demonstrated that reactive oxygen species (ROS) constitute the link between chronic inflammation and neoplasia. Initial experiments on the role of ROS in tumor initiation indicated that oxidative stress directly damages DNA, promoting mutations that favor oncogenic transformation [16,17][16][17].
Non-actinic cutaneous HSA, in turn, occurs more frequently in dogs of non-predisposed breeds that have pigmented skin and thick coats, as well as in those without a history of chronic sun exposure. Therefore, in the histopathological analysis of these tumors, actinic alterations are not identified [11]. Although the origin of non-actinic HSA remains unknown, solar radiation is unlikely to play a role in its development, and the etiopathogenesis is likely similar to that of visceral HAS [18]. Further studies are required to confirm this hypothesis.
Several studies have investigated the etiopathogenesis of this neoplasm by analyzing genomic profiles to identify mutations in specific genes. Similar to visceral HSA, the most frequently mutated gene in canine cutaneous HSA is TP53 [19]. However, it is important to highlight that TP53 mutations are important and frequently found in several cancer subtypes. García-Iglesias et al. [20] found a high expression of the mutated TP53 gene associated with high Ki-67 proliferative activity in 25 dogs with cutaneous HSA, suggesting the association of this gene mutation with neoplastic development. Both studies included patients with dermal and hypodermic HSAs. However, further studies are necessary to elucidate the TP53 role in canine HAS development.
Dysregulation of the PI3K/AKT/mTOR pathway is also very important in canine tumors [21]. Kim et al. [18] evaluated the comparative genomic alteration in human and canine angiosarcomas and found TP53, PIK3CA, PIK3R1, and NRAS in a specific set of canine hemangiosarcomas suggesting these genes dysregulation could be important for tumor development [18].
Mutations in PTEN genes and overexpression of angiogenesis-related growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), have also been identified in cutaneous and subcutaneous HSA [2[10][19][21][22],19,21,22], suggesting participation in the etiopathogenesis of the disease.
Subcutaneous and Muscular HSAemangiosarcoma
Compared with dermal-confined cutaneous HSA, few studies have exclusively evaluated subcutaneous and muscular HSAs. These subtypes account for approximately 6–47% of canine HSAs, affecting middle-aged to elderly dogs (mean age 9 years), with no gender predisposition [2,11,13,23,24,25,26][10][11][13][23][24][25][26].
Due to the scarcity of data in the current literature, it is not yet possible to identify a significant breed predisposition. However, studies have reported a higher incidence of subcutaneous and muscular HSA in golden retrievers, labrador retrievers, and mixed-breed dogs [25,26,27][25][26][27]. Furthermore, unlike actinic cutaneous HSA and similar to non-actinic cutaneous HSA, the development of these subtypes does not seem to correlate with exposure to solar radiation, and their etiopathogenesis may be related to the same factors involved in the development of visceral HSA [13,18,28][13][18][28].
3. Clinical Manifestation and Biological Behavior
Actinic and Non-Actinic Cutaneous HSA
Actinic and Non-Actinic Cutaneous Hemangiosarcoma
The clinical symptoms in dogs with non-visceral HSA vary according to the primary location of the neoplasm and local infiltration. Actinic cutaneous HSA appears more frequently in the ventroabdominal (Figure 2), preputial, and pelvic limb regions and in glabrous, short-haired dogs with a history of chronic sun exposure [2,3,11][2][10][11].
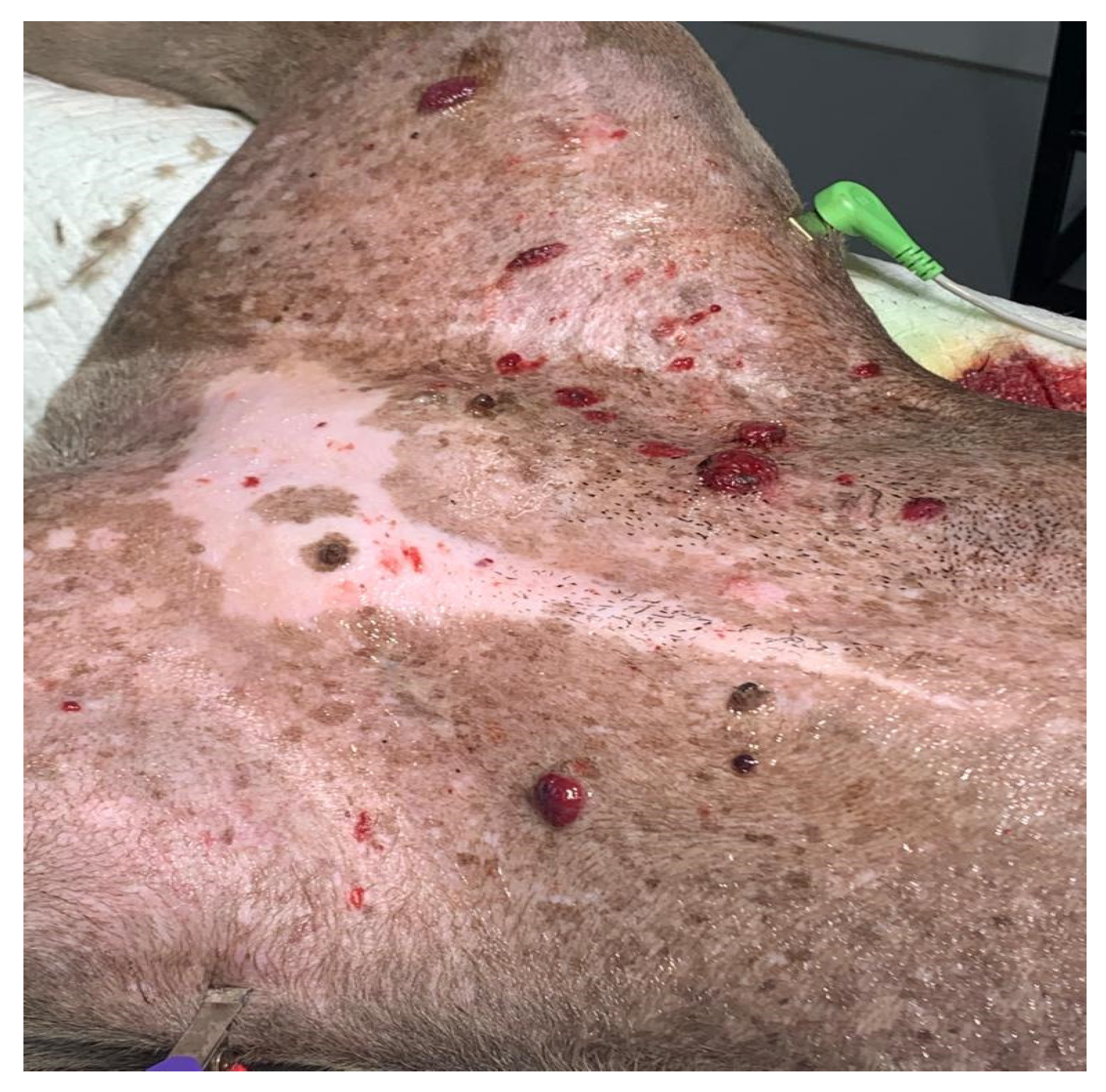
Figure 2.
Multiple actinic lesions in a Pit Bull dog that had a history of chronic sun exposure.
Generally, dermal cutaneous HSA presents as solitary or multiple superficial nodules or papules with a reddish-to-blackish color. Clinical manifestations are usually local and limited to slight intermittent bleeding in the tumor region [1,3][1][2]. Studies have identified a higher incidence (28–35%) of the development of multiple lesions of actinic cutaneous HSA in dogs of predisposed breeds [3,11][2][11].
Despite its exact histological origin, the biological behavior of actinic cutaneous HSA differs from that of non-actinic HSA. In dogs that develop the actinic subtype, the disease tends to manifest in a less aggressive course with a lower probability of metastatic development and longer survival. In contrast, non-actinic cutaneous HSA is associated with a higher metastatic rate and lower survival rate, with a higher incidence in non-predisposed breeds [2,11][10][11].
4. Subcutaneous and Muscular HSAemangiosarcoma
Subcutaneous and muscular HSAs present as more significant, adherent, or mobile than actinic or cutaneous, with a firm-to-soft consistency, and may be associated with ulcerations [1,3,13,25][1][2][13][25].
Unlike the actinic cutaneous subtype, subcutaneous and muscular HSAs do not have an anatomical predilection for their development and may appear in different locations, including the limbs, flank, trunk, scapula, and cervical regions [25,26,27][25][26][27]. Affected animals may develop local pain, lameness, or functional impairment of other structures depending on the anatomical location of the tumor [3,15,26][2][15][26].
Local hemorrhages in subcutaneous tissue and muscles can occur in dogs with advanced HSA [1,26][1][26]. As in visceral HSA, the blood vessels associated with the tumor are deformed and tortuous, leading to blood deprivation for tumor cells and resulting in death. This event causes small foci of vascular rupture, culminating in blood leakage into the subcutaneous or intramuscular space [18].
Subcutaneous and muscular HSAs are naturally more aggressive than the dermal subtype since these subtypes have infiltrative growth involving deeper tissues and greater metastatic capacity [3,26,29][2][26][29]. However, no studies have compared the behavior of non-actinic cutaneous HSA with those of subcutaneous and muscular HSA.
5. Treatment
Local Therapies
Surgery is generally the treatment of choice for dogs with localized non-visceral HSA without evidence of metastatic disease. Therefore, cutaneous, subcutaneous, and muscular subtypes are addressed in the same subtopic [1]. For dogs with cutaneous HSA (stage I), surgical resection with lateral margins of 1–2 cm and deep margins in the fascial plane is recommended. Patients can be cured when complete lesions are removed, particularly in cases of cutaneous HSA with actinic components [3,5,11][2][4][11]. Recent studies have evaluated the application of proportional or “mirrored” lateral margins in mast cell tumors <2 cm, obtaining satisfactory results concerning local control [45,46][30][31]. This approach can also be considered for cutaneous HSA, particularly in cases where multiple small lesions are present. However, further studies are required to better define this criterion for cutaneous HSA. When the tumors are more extensive and infiltrative, which occurs mainly in cases involving the subcutaneous and muscular tissues, more extensive surgeries are necessary and recommend lateral surgical margins of at least 3 cm, similar to those adopted in sarcomas of soft tissues [1,5][1][4]. Widely used in human medicine, transoperative histological evaluation by freezing has gained popularity in veterinary medicine and can be used to confirm the diagnosis of cutaneous HSA, as well as to identify neoplastic cells in the lateral and deep surgical margins, helping to determine the extent of the procedure [47,48][32][33]. Given the scarcity of data in the literature about the rate of lymphatic metastases by HSA, there is limited information about the real benefit of performing lymphadenectomy at the time of surgery. Therefore, lymphadenectomy is recommended only in specific cases in which at least one of the following criteria are present: (1) neoplastic infiltration in a lymph node, detected by previous FNAB; (2) changes in palpation examination (volume, shape, consistency, and/or adherence); and (3) morphological alterations identified through imaging tests (abdominal ultrasound or computed tomography). Obtaining wide margins becomes difficult when developing multiple large tumors and/or in complex anatomical sites, which may require more radical surgical procedures [1,3][1][2]. Alternatively, for these cases, less extensive surgical approaches can be associated with local and systemic therapies such as electrochemotherapy, radiotherapy, or adjuvant chemotherapy [27,49,50][27][34][35]. The use of radiotherapy for the treatment of dogs with cutaneous HSA is uncommon, and only a few studies have evaluated its clinical benefits. Studies published so far suggest the use of palliative radiotherapy in cases of unresectable tumors, with the aim to reduce pain, possible bleeding, and tumor progression; post-surgical radiotherapy is also used for the treatment of compromised margins [1,26,28,50][1][26][28][35]. Hillers et al. (2007) [50][35] evaluated the effect of palliative radiotherapy (6–24 Gy) in 20 dogs with unresectable subcutaneous and intramuscular HSA with or without surgery and chemotherapy and found an overall response rate of 70% (10 PR and 4 CR); however, there was no increase in overall survival time. The efficacy of radiotherapy in the treatment of microscopic disease after incomplete surgical resection in dogs with subcutaneous and intramuscular HSA remains controversial. Bulakowski et al. (2008) [25] administered post-surgical radiotherapy to five dogs and found a short duration of response, and local recurrence occurred in three of these five dogs. Shiu et al. (2011) [26] found that the three dogs treated with radiotherapy for the same purpose had no local recurrence. Larger prospective studies are needed to determine the effectiveness of radiotherapy in the local control of subcutaneous and intramuscular HSA. Recently, electrochemotherapy (ECT) has emerged as an alternative therapy for the local ablative treatment of different neoplastic types [51[36][37][38],52,53], and its application has already been standardized in Europe for cutaneous and subcutaneous tumors of different histological types in humans, canines, and felines [51][36]. ECT consists of a chemotherapeutic drug, generally of a hydrophilic nature, whose penetration through the plasma membrane is limited, used with electrical pulses; the electrical pulses provide a temporary and reversible increase in the permeability of the target cell membrane, maximizing the intracellular concentration of the previously applied chemotherapy, and consequently, its cytotoxic effect [54,55][39][40]. The main antineoplastic drugs used are bleomycin and cisplatin, which can be administered intravenously, intratumorally, or intratumorally. Bleomycin (BLM) is the most commonly used chemotherapeutic in ECT protocols because the internalization of its molecules is enhanced by approximately 300 to 700 times in the intracellular environment [56][41]. BLM has the possibility of intravenous administration, allowing its homogeneous distribution in the area to be treated and providing unique selectivity. This is attributed to its cell death mechanism, which provides selective destruction of cells that are in high mitotic activity (tumor cells) and allows the treatment of margins, preserving healthy tissue and eliminating possible microscopic neoplastic foci present there [51,57][36][42]. In addition to its cytotoxic antitumor effect, ECT exerts a hemostatic action on the tumor vasculature through local vasoconstriction. This effect promotes a reduction in intraoperative bleeding when used as an adjunct to surgery and as a monotherapy in multiple and hemorrhagic lesions [58][43]. Campana et al. (2019) [59][44] evaluated 20 human patients with 51 target lesions who underwent ECT in cutaneous HSA in advanced stages, in which surgery became unfeasible due to local spread. CR was observed in 61%, PR in 22%, DE in 18%, and DP in 2% of injuries. CR was observed in 40% (8/20), PR in 40% (8/20), DE in 15% (3/20), and DP in 5% (1/20) of patients. Patients who achieved CR had considerable local control with an average progression-free time of 10.9 months. Regarding the antivascular effect, a reduction in bleeding was observed in 93% of the patients (13/14) who had an ulcerated tumor, providing significant palliative benefits and promoting quality of life. However, 35% of patients (n = 7) had tumor recurrence after 3.4 months. Palliative ECT has been used to improve quality of life and life expectancy. Several studies have already evaluated the effectiveness of ECT in different types of canine sarcomas, mostly soft tissue sarcomas (STMs), with promising results [60,61,62,63,64][45][46][47][48][49]. However, owing to the small population of dogs with cutaneous HSA in these studies, it is still impossible to comprehensively determine the effectiveness of ECT in these tumors. In most studies performed with ECT in cutaneous hemangiosarcomas, the therapy is used almost exclusively as an adjuvant to surgical treatment and has a limited response to its use alone [63][48]. However, despite the lack of a large sample group, the clinical benefit of ECT therapy seems promising in terms of local control and decreased recurrence when used together with surgery [63][48]. In a recent study of 30 dogs with soft tissue sarcoma, only 2 had a diagnosis of cutaneous HSA with a DFI of 1053 and 366 days, since the second dog died due to recurrence and metastasis at that time [63][48]. Spugnini et al. [64][49] observed 22 dogs with soft tissue sarcoma, and 3 were diagnosed with HSA in limited anatomical regions regarding the practical surgical approach (limbs, pelvis, and thorax). All patients had local control but a short survival time (30, 60, and 150 days) with recurrence, metastasis, and death from splenic HSA. The distribution of intratumoral BLM is affected by the intrinsic vascularization of the tumor. Therefore, the results of electroporation associated with chemotherapy combinations vary. In a study of human patients, it was observed that patients diagnosed with cutaneous angiosarcoma had the highest concentrations of BLM (819 mg/g); however, the tumor presented tumor progression and had no response to ECT [65[50][51],66], showing a difference in perfusion, vascularization, and response in different tumor types. As observed in human patients, cutaneous toxicity is also observed when using ECT alone, with varying degrees of cutaneous ulceration [59][44] (personal communication) (Figure 3 and Figure 4).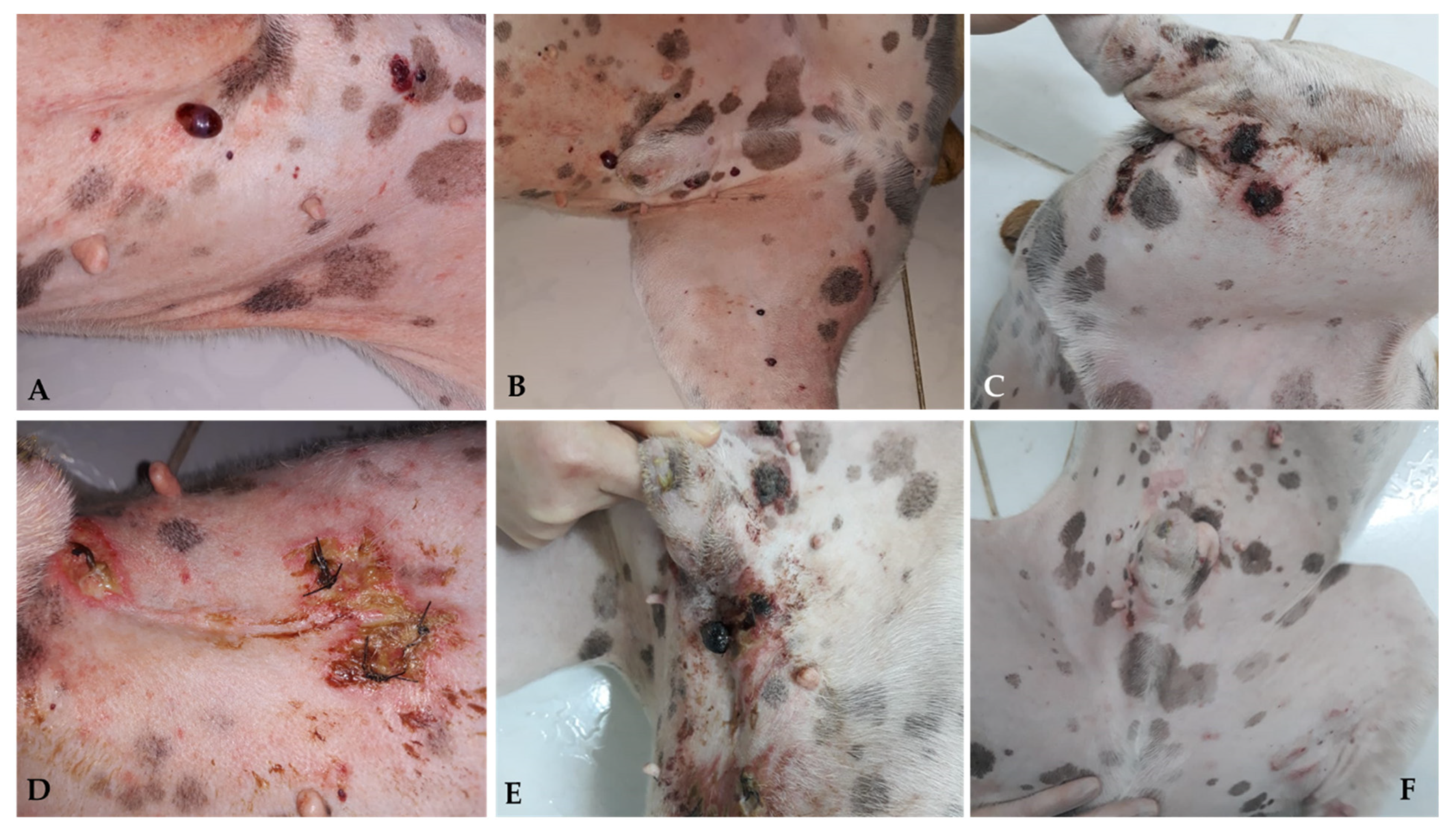
Figure 3. Canine patient diagnosed with multiple cutaneous HSA nodules; ECT with systemic BLM was administered. Multiple skin lesions are observed in the ventral abdomen and medial surface of the pelvic limb (A,B). Crust and ulceration 7 days after ECT (C–E). Complete remission 30 days after ECT with areas of scar tissue (F).
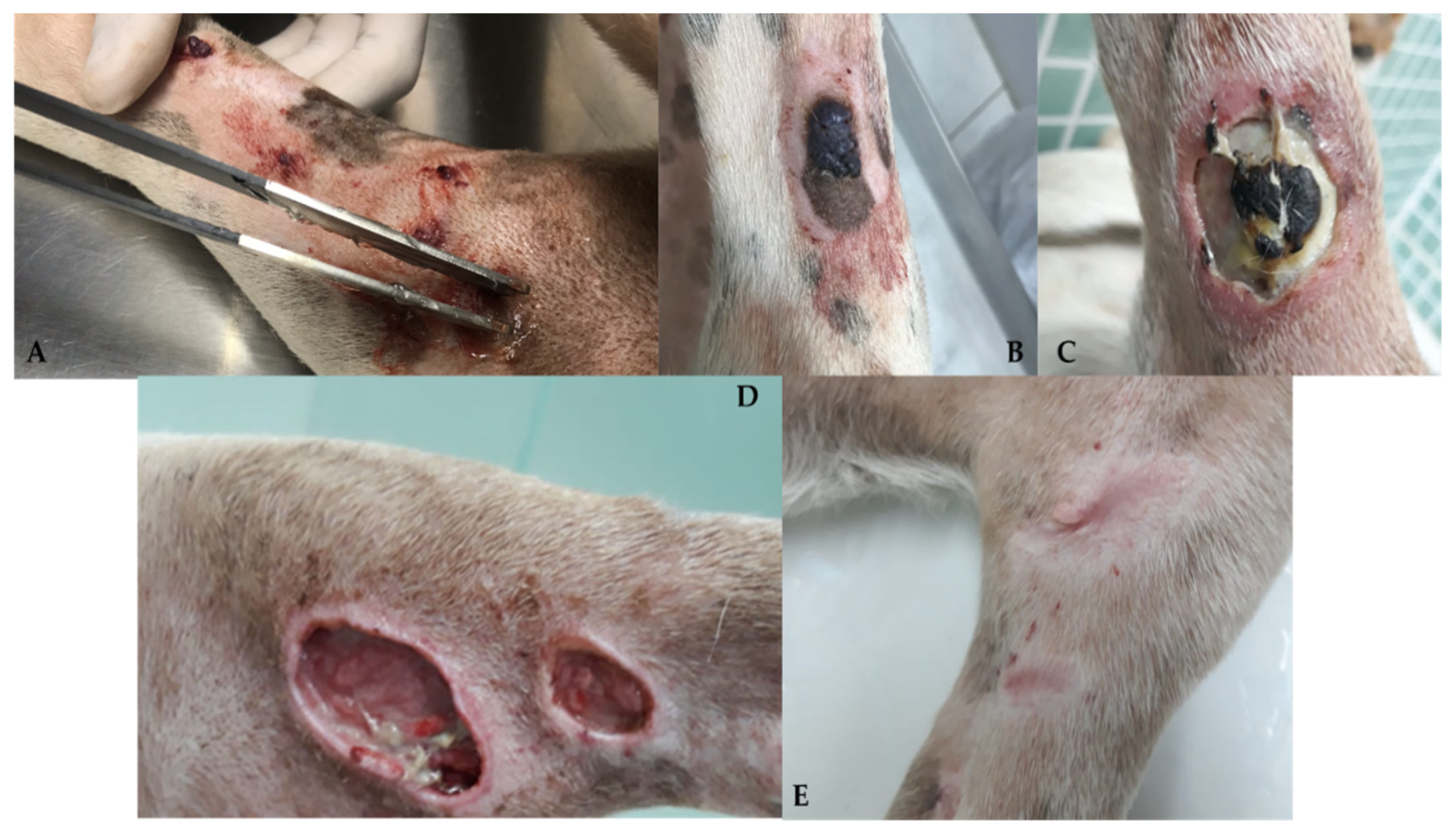
Figure 4. Canine patient diagnosed with multiple cutaneous HSA who underwent ECT with systemic BLM. Multiple skin lesions are observed in the region of the pelvic limb (A). Crust and ulceration 7 days after ECT (B,C). Necrosis and tissue loss 15 days after ECT (D). Complete remission 30 days after ECT with areas of scar tissue (E).
6. Systemic Therapies
6.1. Actinic and Non-Actinic Cutaneous HSA
6.1. Actinic and Non-Actinic Cutaneous Hemangiosarcoma
Unlike in cases of visceral HSA, surgical treatment is considered curative for dogs with actinic cutaneous HSA, confined to the dermis, and without metastatic evidence (stage I), with no indication of adjuvant chemotherapy for these patients. However, for cases of non-actinic HSA, there is still no consensus regarding the indications for adjuvant chemotherapy. Given the more aggressive and metastatic behavior of non-actinic HSA, studies must be conducted to determine the clinical benefits of systemic therapy in these patients [11].6.2. HSA Subcutaneous and Muscular
6.2. Hemangiosarcoma Subcutaneous and Muscular
There is greater consensus in the literature regarding the indications for adjuvant chemotherapy in subcutaneous and muscular HSA [5][4]. This recommendation is based on the more aggressive biological behavior inherent in these subtypes, both because of their infiltrative nature, making surgical resection and local control more complex, and because of their significant metastatic potential, resulting in shorter survival times and, consequently, worse prognoses [3,11,26,69][2][11][26][54]. Similar to visceral HSA, doxorubicin is the most frequently used antineoplastic agent for subcutaneous and muscular subtypes and can be administered alone or in combination with other drugs [23,24,25,32,69][23][24][25][54][55]. Retrospective studies that evaluated the use of chemotherapy in animals with subcutaneous and intramuscular HSAs found median survival rates between 210 and 1189 days (Table 21).Table 21. Chemotherapy protocols evaluated in canine subcutaneous and intramuscular HSA, type of surgery, and median survival.
| Chemotherapy Protocol | N | HSA Type | Surgery | Mean Survival Time (Days) |
|---|---|---|---|---|
| VAC Protocol (Hammer et al., 1991) [32][55] |
4 | Subcutaneous | Incomplete resection (n = 4) | 436 |
| AC Protocol (Sorenmo et al., 1993) [23] |
5 | Subcutaneous | Complete resection (n = 2); Incomplete resection (n = 3) |
240 |
| Doxorrubicin +/− Cyclofosfamide (Bulakowski et al., 2008) [25] |
21 | Subcutaneous (n = 17) Intramuscular (n = 4) |
Complete resection (in the first surgery—n = 11; in the second surgery—n = 5); Adjuvant radiotherapy after incomplete resection (n = 5) a |
1189 (subcutaneous) 272 (intramuscular) b |
| Doxorrubicin +/− ciclofosfamide, vincristine or lomustine (Shiu et al., 2011) [26] |
36 | Subcutaneous (n = 55) Intramuscular (n = 16) |
Complete resection (n = 18); Incomplete resection (n = 18) | 212 (subcutaneous) 136 (intramuscular) c |
a Adequate local control achieved for all dogs. b Presence of statistically significant difference between groups (p = 0.002). c Absence of statistically significant difference between groups (p = 0.268).
Hammer et al. (1991) [32][55] treated four dogs with subcutaneous HSAs with incomplete surgical resection with the VAC protocol, resulting in a median survival of 436 days. Sorenmo et al. (1993) [23] evaluated the AC protocol in 5 dogs with subcutaneous HSAs (complete resection in 2/5 dogs), which achieved a median survival of 240 days. Bukowski et al. (2008) [25] evaluated chemotherapy in only dogs with subcutaneous and intramuscular HSA (n = 21) after adequate local surgical control, using doxorubicin with or without cyclophosphamide; 17 patients with subcutaneous HSA had significantly higher median survival than those with intramuscular HSA (1189 vs. 272 days, respectively).
Shiu et al. (2011) [26] studied 55 dogs with subcutaneous HSAs; 17 received adjuvant chemotherapy (protocols with doxorubicin combined with other drugs) after complete surgical resection and had a median survival of 246 days. The type of HSA and adjuvant treatment did not influence the clinical outcomes of patients. However, dogs that underwent total surgical excision of the lesions had a significantly higher median survival and progression-free time than those with incomplete resection.
Prospective studies in dogs with stage II and III HSA are needed to better determine the therapeutic benefit of adjuvant chemotherapy. However, given the more advanced staging and more aggressive course, the authors of this consensus recommend using adjuvant chemotherapy in these cases, with doxorubicin as the primary drug.
Aggressive and infiltrative subcutaneous and intramuscular HSAs can be unresectable, making surgical treatment unfeasible in affected animals. For these cases, chemotherapy can be instituted in two different situations: (1) neoadjuvant to surgery (pre-surgical) to reduce tumor dimensions to make surgical excision possible, either with curative or palliative intent, or (2) purely palliatively, without association with surgery, to offer comfort to patients with advanced and unresectable tumors.
In this context, Wiley et al. (2010) [27] evaluated the benefits of chemotherapy with or without doxorubicin in 18 canine patients with unresectable subcutaneous HSA. The dogs received surgical treatment after chemotherapy (neoadjuvant scenario, n = 5) or chemotherapy alone (palliative scenario, n = 13). Doxorubicin was administered in all protocols as a single drug (12/18) or in combination with other chemotherapeutics (6/18) every 2 or 3 weeks. The overall response rate ranged from 38 to 44%, and tumor excision was achieved with wide margins in 80% of the patients who underwent surgery after chemotherapy. Although the response was short-lived, neoadjuvant chemotherapy provided clinical benefits for these patients, and surgical excision was associated with a longer progression-free time.
6.3. Other Therapies
Considering the vascular nature of HSAs, antiangiogenic therapy is a potential therapeutic target for these tumors in dogs, whether in their visceral or cutaneous form [12,70][12][56]. Its use has been widely investigated, mainly in splenic HSA, and studies are needed to assess its clinical benefit in canine cutaneous HSA [12,70,71][12][56][57]. In recent years, the search for molecular therapeutic targets in visceral and non-visceral HSAs has gained prominence in veterinary medicine [1]. Nóbrega et al. (2019) [2][10] identified high expression of COX-2 and VEGF in 60 dogs with cutaneous HSA, suggesting that these may represent potential therapeutic targets. Further clinical studies are required to prove that their inhibition leads to antitumor effects. Photodynamic therapy has also emerged, with limitations in surgical resection, either by extension or by several lesions. Rocha et al. (2019) [72][58] evaluated the use of this therapy in eight dogs with cutaneous HSAs and achieved complete remission in 90% of cases. As this is a new therapy, prospective studies with more animals are needed. The association between actinic dermatosis and cutaneous hemangiosarcoma can be explored therapeutically using natural photoprotectors such as omega-3 fatty acids, vitamins, and polyphenols [73,74][59][60]. Omega-3 polyunsaturated fatty acids (AGPs) primarily comprise α-linoleic acid (ALA), docoHSAexaenoic acid (DHA), and eicosapentaenoic acid (EPA) and come directly from foods and/or dietary supplements enriched with omega-3 fish oil. Omega-3 AGPs exert anti-inflammatory effects. Studies involving its metabolites, such as resolverins and maresins, have revealed potent anti-inflammatory, analgesic, and antineoplastic activities, mainly by reducing the production of cytokines/chemokines derived from neoplastic cells (TNF-α, IL-6, CXCL10, and MCP-1) and CD11bþLy6G myeloid cells induced by tumor mediators and nociception. A decrease in ROS production and improvement in the phagocytic activity of macrophages were also observed in one study [75,76,77,78][61][62][63][64]. Treatment with maresin 1, a lipid mediator derived from omega-3 AGP, was associated with protection against UVB radiation in hairless mice. The results showed less inflammation and oxidative stress in the treated animals, which was related to the inhibition of edema, keratinocyte apoptosis, and the presence of mast cells. Furthermore, the lower production of metalloproteinases 9 (MMP9) and the consequent degradation of collagen fibers induced by radiation were attributed to the administration of maresin 1 [16]. Vitamins B3 and C and natural polyphenols, including cannabis derivatives, have been associated with the photoprotective activity of the skin [73,79,80,81][59][65][66][67]. A study that exposed rats to UVA and UVB radiation demonstrated the protective effect of phytocannabinoid-cannabidiol (CBD) on the lipid metabolism of keratinocytes, reducing 4-HNE and 8-isoPGF2α lipid peroxidation products. This experiment also demonstrated that CBD increased the transcriptional activity of Nrf2 and the expression of its inhibitor, Bach1, and prevented the UVA/UVB-induced increase in the expression of Nrf2 activators p21, p62, p38, and KAP1, and pro-inflammatory factors, such as NF-κB and TNF-α [80][66].References
- Mullin, C.; Clifford, C.A. Miscellaneous tumours: Hemangiosarcoma. In Withrow and MacEwen’s Small Animal Clinical Oncology, 5th ed.; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2020; pp. 773–778. ISBN 978-0-323-59496-7.
- Ward, H.; Fox, L.E.; Calderwood-Mays, M.B.; Hammer, A.S.; Couto, C.G. Cutaneous hemangiosarcoma in 25 dogs: A retrospective study. Vet. Intern. Med. 1994, 8, 345–348.
- Tinsley, A. Canine Hemangiosarcoma: A Certainly Less Than Ideal, Very Ugly Cancer. Preprints 2020, 1, 1–14.
- Griffin, M.A.; Culp, W.T.N.; Rebhun, R.B. Canine and feline haemangiosarcoma. Vet. Rec. 2021, 585, e585.
- Flores, M.M.; Panziera, W.; Kommers, G.D.; Irigoyen, L.F.; Barros, C.S.L.; Fighera, R.A. Aspectos epidemiológicos e anatomopatológicos do hemangiossarcoma em cães: 40 casos (1965–2012). Pesq. Vet. Bras. 2012, 32, 1319–1328.
- Soares, N.P.; Medeiros, A.A.; Szabó, M.P.J.; Guimarães, E.C.; Fernandes, L.G.; dos Santos, T.R. Hemangiomas e Hemangiossarcomas em cães: Estudo retrospectivo de 192 casos (2002–2014). Ciênc. Anim. Bras. 2017, 18, 1–10.
- Andrade, R.L.; Oliveira, D.M.; Dantas, A.F.M.; de Souza, A.P.; da Nóbrega, P.I.N.; Riet-Correa, F. Tumores de cães e gatos diagnosticados no semiárido da Paraíba. Pesq. Vet. Bras. 2012, 32, 1037–1040.
- Alves, D.S.; Calvaca, M.B.; Fonseca-Alves, C.E. A Critical Review of the risk factors associated with Canine Squamous Cell Carcinoma development. Braz. J. Vet. Pathol. 2022, 15, 1–10.
- DSA-INPE: Divisão de Satélites e Sistemas Ambientais, Instituto Nacional de Pesquisas Espaciais, Índice Ultravioleta Home page. Available online: http://satelite.cptec.inpe.br/uv (accessed on 20 March 2022).
- Nóbrega, D.F.; Sehaber, V.F.; Madureira, R.; Bracarense, A.P.F.R.L. Canine cutaneous haemangiosarcoma: Biomarkers and survival. J. Comp. Pathol. 2019, 166, 87–96.
- Szivek, A.; Burns, R.E.; Gericota, B.; Affolter, V.K.; Kent, M.S.; Rodriguez, C.O., Jr.; Skorupski, K.A. Clinical outcome in 94 cases of dermal haemangiosarcoma in dogs treated with surgical excision: 1993–2007. Vet. Comp. Oncol. 2012, 10, 65–73.
- Vilar-Saavedra, P.; Kitchell, B.E. Sunlight-Induced Skin Cancer in Companion Animals. In Skin Cancer—A Practical Approach, 1st ed.; Baldi, A., Pasquali, P., Spugnini, E.P., Eds.; Humana Press: London, UK, 2014; pp. 499–514. ISBN 978-1-4614-7357-2.
- Hargis, A.M.; Ihrke, P.J.; Spangler, W.L.; Stannard, A.A. A retrospective clinicopathologic study of 212 dogs with cutaneous hemangiomas and hemangiosarcomas. Vet. Pathol. 1992, 29, 316–328.
- Nikula, K.J.; Benjamin, S.A.; Angleton, G.M.; Saunders, W.J.; Lee, A.C. Ultraviolet radiation, solar dermatosis, and cutaneous neoplasia in beagle dogs. Radiat. Res. 1992, 129, 11–18.
- Fernandes, S.C.; De Nardi, A.B. Hemangiossarcomas. In Oncologia de Cães e Gatos, 2nd ed.; Daleck, C.R., De Nardi, A.B., Eds.; Roca: Rio de Janeiro, RJ, Brazil, 2016; pp. 776–796. ISBN 9788527729918.
- Cezar, T.L.C.; Martinez, R.M.; da Rocha, C.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with maresin 1, a docoHSAexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci. Rep. 2019, 9, 3062.
- Nediani, C.; Dinu, M. Oxidative Stress and Inflammation as Targets for Novel Preventive and Therapeutic Approaches in Non-Communicable Diseases II. Antioxidants 2022, 11, 824.
- Kim, J.H.; Graef, A.J.; Dickerson, E.B.; Modiano, J.F. Pathobiology of hemangiosarcoma in dogs: Research advances and future perspectives. Vet. Sci. 2015, 2, 388–405.
- Wong, K.; Ludwig, L.; Krijgsman, O.; Adams, D.J.; Wood, G.A.; van der Weyden, L. Comparison of the oncogenomic landscape of canine and feline hemangiosarcoma shows novel parallels with human angiosarcoma. Dis. Model. Mech. 2021, 14, dmm049044.
- García-Iglesias, M.J.; Cuevas-Higuera, J.L.; Bastida-Sáenz, A.; de Garnica-García, M.G.; Polledo, L.; Perero, P.; González-Fernández, J.; Fernández-Martínez, B.; Pérez-Martínez, C. Immunohistochemical detection of p53 and pp53 Ser392 in canine hemangiomas and hemangiosarcomas located in the skin. BMC Vet. Res. 2020, 16, 239.
- Rivera-Calderón, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Vasconcelos, R.O.; Laufer-Amorim, R. p-mTOR, p-4EBP-1 and eIF4E expression in canine prostatic carcinoma. Res. Vet. Sci. 2019, 122, 86–92.
- Yonemaru, K.; Sakai, H.; Murakami, M.; Yanai, T.; Masegi, T. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and their receptors (flt-1, flk-1, and flg-1) in canine vascular tumors. Vet. Pathol. 2006, 43, 971–980.
- Sorenmo, K.U.; Jeglum, K.A.; Helfand, S.C. Chemotherapy of canine hemangiosarcoma with doxorubicin and cyclophosphamide. J. Vet. Int. Med. 1993, 7, 370–376.
- Ogilvie, G.K.; Powers, B.E.; Mallinckrodt, C.H.; Withrow, S.J. Surgery and doxorubicin in dogs with hemangiosarcoma. J. Vet. Int. Med. 1996, 10, 379–384.
- Bulakowski, E.J.; Philibert, J.C.; Siegel, S.; Clifford, C.A.; Risbon, R.; Zivin, K.; Cronin, K.L. Evaluation of outcome associated with subcutaneous and intramuscular hemangiosarcoma treated with adjuvant doxorubicin in dogs: 21 cases (2001–2006). J. Am. Vet. Med. Assoc. 2008, 233, 122–128.
- Shiu, K.B.; Flory, A.B.; Anderson, C.L.; Wypij, J.; Saba, C.; Wilson, H.; Kurzman, I.; Chun, R. Predictors of outcome in dogs with subcutaneous or intramuscular hemangiosarcoma. J. Am. Vet. Med. Assoc. 2011, 238, 472–479.
- Wiley, J.L.; Rook, K.A.; Clifford, C.A.; Gregor, T.P.; Sorenmo, K.U. Efficacy of doxorubicin-based chemotherapy for non-resectable canine subcutaneous haemangiosarcoma. Vet. Comp. Oncol. 2010, 8, 221–233.
- Smith, A.N. Hemangiosarcoma in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 533–552.
- Schultheiss, P.C. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J. Vet. Diagn. 2004, 16, 522–526.
- Chu, M.L.; Hayes, G.M.; Henry, J.G.; Oblak, M.L. Comparison of lateral surgical margins of up to two centimeters with margins of three centimeters for achieving tumor-free histologic margins following excision of grade I or II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 2020, 256, 567–572.
- Saunders, H.; Thomson, M.J.; O’Connell, K.; Bridges, J.P.; Chau, L. Evaluation of a modified proportional margin approach for complete surgical excision of canine cutaneous mast cell tumours and its association with clinical outcome. Vet. Comp. Oncol. 2020, 19, 604–615.
- Whitehair, J.G.; Griffey, S.M.; Olander, H.J.; Vasseur, P.B.; Naydan, D. The Accuracy of Intraoperative Diagnoses Based on Examination of Frozen Sections A Prospective Comparison with Paraffin-Embedded Sections. Vet. Surg. 1993, 22, 255–259.
- Hashmi, A.A.; Riaz, R.; Zia, S.; Shahid, H.; Malik, U.A.; Khan, R.; Irfan, M.; Shamail, F.; Zia, F.; Asif, M.G. Impact of Histological Type and Grade on the Diagnostic Accuracy of Intraoperative Frozen Section for Detecting Breast Cancer Metastasis to Axillary Sentinel Lymph Nodes. Cureus 2021, 13, e16146.
- Tozon, N.; Sersa, G.; Cemazar, M. Electrochemotherapy: Potentiation of local antitumor effectiveness of cisplatin in dogs and cats. Anticancer Res. 2001, 21, 2483–2488.
- Hillers, K.R.; Lana, S.E.; Fuller, C.R.; LaRue, S.M. Effects of palliative radiation therapy on nonsplenic hemangiosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 2007, 43, 187–192.
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. EJC Suppl. 2006, 4, 14–25.
- Rangel, M.M.; Luz, J.; Oliveira, K.D.; Ojeda, J.; Freytag, J.O.; Suzuki, D.O. Electrochemotherapy in the treatment of neoplasms in dogs and cats. Austral J. Vet. Sci. 2019, 51, 45–51.
- Spugnini, E.P.; Baldi, A. Electrochemotherapy in veterinary oncology: State-of-the-art and perspectives. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 967–979.
- Mir, L.M. Bases and rationale of the electrochemotherapy. EJC Suppl. 2006, 4, 38–44.
- Escoffre, J.M.; Rols, M.P. Electrochemotherapy: Progress and prospects. Curr. Pharm. Des. 2012, 18, 3406–3415.
- Mir, L.M.; Orlowski, S.; Belehradek, J.J.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer 1991, 27, 68–72.
- Tounekti, O.; Pron, G.; Belehradek, J.; Mir, L.M. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res. 1993, 53, 5462–5496.
- Sersa, G.; Jarm, T.; Kotnik, T.; Coer, A.; Podkrajesk, M.; Sentjurc, M.; Miklavcic, D.; Kadivec, M.; Kranjc, S.; Secerov, A.; et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br. J. Cancer 2008, 98, 388–398.
- Campana, L.G.; Kis, E.; Bottyán, K.; Orlando, A.; de Terlizzi, F.; Mitsala, G.; Careri, R.; Curatolo, P.; Snoj, M.; Sersa, G.; et al. Electrochemotherapy for advanced cutaneous angiosarcoma: A European register-based cohort study from the International Network for Sharing Practices of electrochemotherapy (InspECT). Int. J. Surg. 2019, 72, 34–42.
- Spugnini, E.P.; Porrello, A. Potentiation of chemotherapy in companion animals with spontaneous large neoplasms by application of biphasic electric pulses. J. Exp. Clin. Cancer Res. 2003, 22, 571–580.
- Spugnini, E.P.; Citro, G.; D’Avino, A.; Baldi, A. Potential role of electrochemotherapy for the treatment of soft tissue sarcoma: First insights from preclinical studies in animals. Int. J. Biochem. Cell Biol. 2008, 40, 159–163.
- Spugnini, E.P.; Vincenzi, B.; Betti, G.; Cordahi, F.; Dotsinsky, I.; Mudrov, N.; Citro, G.; Baldi, A. Surgery and electrochemotherapy of a high-grade soft tissue sarcoma in a dog. Vet. Rec. 2008, 162, 186–188.
- Spugnini, E.P.; Vincenzi, B.; Amadio, B.; Baldi, A. Adjuvant electrochemotherapy with bleomycin and cisplatin combination for canine soft tissue sarcomas: A study of 30 cases. Open Vet. J. 2019, 9, 88–93.
- Torrigiani, F.; Pierini, A.; Lowe, R.; Simčič, P.; Lubas, G. Soft tissue sarcoma in dogs: A treatment review and a novel approach using electrochemotherapy in a case series. Vet. Comp. Oncol. 2019, 17, 234–241.
- Spugnini, E.P.; Vincenzi, B.; Citro, G.; Santini, D.; Dotsinsky, I.; Mudrov, N.; Montersarchio, V.; Laieta, M.T.; Esposito, V.; Baldi, A. Adjuvant electrochemotherapy for the treatment of incompletely excised spontaneous canine sarcomas. Vivo 2007, 21, 819–822.
- Groselj, A.; Bosnjak, M.; Krzan, M.; Kosjek, T.; Bottyán, K.; Plesnik, H.; Jamsek, C.; Cemazar, M.; Kis, E.; Sersa, G. Bleomycin Concentration in Patients’ Plasma and Tumors after Electrochemotherapy. A Study from InspECT Group. Pharmaceutics 2021, 13, 1324.
- de Queiroz, G.F.; Matera, J.M.; Dagli, M.L.Z. Clinical study of cryosurgery efficacy in the treatment of skin and subcutaneous tumors in dogs and cats. Vet. Surg. 2008, 37, 438–443.
- Laus, J.L.; Ortiz, J.P.D.; Brito, F.L.C.; Lisbão, C.B.S.; Silva, V.A., Jr.; Maia, F.C.L. Hemangiosarcoma of the nictitant membrane in a Brazilian Fila dog: Case report. Arq. Bras. Med. Vet. Zootec. 2008, 60, 1413–1417.
- Alvarez, F.J.; Hosoya, K.; Lara-Garcia, A.; Kisseberth, W.; Couto, G. VAC Protocol for Treatment of Dogs with Stage III Hemangiosarcoma. J. Am. Anim. Hosp. Assoc. 2013, 49, 370–377.
- Hammer, A.S.; Couto, C.G.; Filppi, J.; Getzt, D.; Shank, K. Efficacy and toxicity of VAC chemotherapy (vincristine doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. J. Vet. Int. Med. 1991, 5, 160–166.
- Finotello, R.; Henriques, J.; Sabattini, S.; Stefanello, D.; Felisberto, R.; Pizzoni, S.; Ferrari, R.; Marconato, L. A retrospective analysis of chemotherapy switch suggests improved outcome in surgically removed, biologically aggressive canine haemangiosarcoma. Vet. Comp. Oncol. 2017, 15, 493–503.
- Treggiari, E.; Borrego, J.F.; Gramer, I.; Valenti, P.; Harper, A.; Finotello, R.; Toni, C.; Laomedonte, P.; Romanelli, G. Retrospective comparison of first-line adjuvant anthracycline vs metronomic-based chemotherapy protocols in the treatment of stage I and II canine splenic haemangiosarcoma. Vet. Comp. Oncol. 2020, 18, 43–51.
- Rocha, M.S.T.; Lucci, C.M.; dos Santos, J.A.; Longo, J.P.F.; Muehlmann, L.A.; Azevedo, R.B. Photodynamic therapy for cutaneous hemangiosarcoma in dogs. Photodiagnosis Photodyn. Ther. 2019, 27, 39–43.
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958.
- Souyoul, S.A.; Saussy, K.P.; Lupo, M.P. Nutraceuticals: A Review. Dermatol. Ther. 2018, 8, 5–16.
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2020, 11, 623052.
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018, 215, 115–140.
- Takamiya, R.; Fukunaga, K.; Arita, M.; Miyata, J.; Seki, H.; Minematsu, N.; Suematsu, M.; Asano, K. Resolvin E1 maintains macrophage function under cigarette smoke-induced oxidative stress. FEBS Open Bio 2012, 2, 328–333.
- Ye, Y.; Scheff, N.N.; Bernabé, D.; Salvo, E.; Ono, K.; Liu, C.; Veeramachaneni, R.; Viet, C.T.; Viet, D.T.; Dolan, J.C.; et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology 2018, 139, 182–193.
- Fania, L.; Mazzanti, C.; Campione, E.; Candi, E.; Abeni, D.; Dellambra, E. Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. Int. J. Mol. Sci. 2019, 20, 5946.
- Jastrząb, A.; Jarocka-Karpowicz, J.; Markowska, A.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Antioxidant and Anti-inflammatory Effect of Cannabidiol Contributes to the Decreased Lipid Peroxidation of Keratinocytes of Rat Skin Exposed to UV Radiation. Antioxidants 2020, 9, 21.
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819.
More
