Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Javier Ordonez and Version 2 by Conner Chen.
Seaweeds are mostly used in the wastewater treatment for removing toxic heavy metals.
- bioremediation
- macroalgae
- mining effluents
- heavy metals
1. Heavy Metals Biosorption by Algae
In the context of biosorption, seaweed is mostly used in the wastewater treatment for reducing or removing toxic heavy metal contents [1][51]. The removal of heavy metals from aquatic courses constitutes a relevant environmental challenge today due to the toxic nature of these elements for living organisms [2][60]. Some heavy metals are toxic and carcinogenic, even in tiny concentrations, and they are non-biodegradable and can easily accumulate in living organisms. The heavy metal accumulation in soils and groundwaters is a growing concern; the main anthropogenic sources are mining operations, smelters, the paint industry, fertilizers, leather tanning, electroplating, alloy and battery manufacturing [3][7].
When evaluating the most studied metals for biosorption with algae, cadmium has the highest number of publications, with approximately 23% of reports based on how toxic it is in the environment and its critical removal. Likewise, it is an indicator that biotechnological methods represent a competitive alternative to traditional processes. Subsequently, copper and lead (20% and 19%, respectively) are found, which are heavy metals highly diffused by mining and industrial operations. The biosorption of more abundant metals, such as iron (3%), has been less studied because the methods of precipitation by neutralizing agents are quite effective and cheap, which does not support a sustained motivation for their investigation (Figure 14).
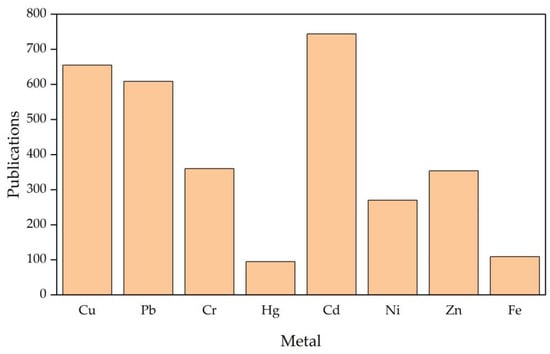
Figure 14. Profile of investigations reported in the WOS database on biosorption of different heavy metals with alga biomasses. A publication can address more than one metal, so the number of articles should be taken as a reference and not as an exact account of each category.
Research on algal biosorption is concentrated on brown algae due to the aforementioned metal-affinity properties. However, the review of articles studying green and red algae to capture metals is increasingly recurrent (Table 13).
Table 13.
Seaweeds studied in biosorption and the metals considered.
| Type | Species | Metal | Refs. |
|---|---|---|---|
| Brown | Hydroclathrus clathratus | Pb, Cr | [4][61] |
| Cystoseira barbata | Pb, Cr | [4][61] | |
| Macrocystis pyrifera | Zn, Cd, Ni | [5][6][62,63] | |
| Fucus vesiculosus | Cd, Pb, Cu | [7][58] | |
| Sargassum filipendula | Cd, Zn | [8][64] | |
| Undaria pinnatifida | Hg | [6][63] | |
| Sargassum sp. | Pb, Cu | [9][65] | |
| Lessonia nigrescens | Cu | [10][66] | |
| Red | Gracilaria chilensis | Cu | [11][39] |
| Gracilaria fisheri | Cd, Cu | [12][67] | |
| Kappaphycus alvarezii | Cr, Ni, Cu | [13][68] | |
| Ceramium virgatum | Cd | [14][69] | |
| Callithamnion corymbosum | Cu, Co, Zn | [15][70] | |
| Green | Codium vermilara | Cu | [16][71] |
| Chlorella vulgaris | Cu | [17][72] |
2. Brown Seaweeds
Chromium, nickel, copper, arsenic, cadmium, mercury and lead are globally alarming heavy metals [18][73]. Pb and Cr are some of the most frequent toxic cations in wastewater. Ali et al. [4][61] studied two brown seaweed species Hydroclathrus clathratus and Cystoseira barbata for Pb and Cr biosorption, concluding that metal concentration had an inverse effect on the metal uptake. Maximum biosorption efficiency was achieved at 120 min, pH 5 and 10 g/L of algae. The maximum uptakes of Pb and Cr biosorption are 4.97, 7.19 mg/g on H. clathratus and 4.61, 7.30 mg/g on C. barbata, respectively.
Plaza Cazón et al. [5][62] used Macrocystis pyrifera to remove zinc and cadmium from mono- and bimetallic solutions, demonstrating in both cases goods uptake capacities, which were similar to other studies performed with Sargassum filipendula, Gymnogongrus torulosus and Fucus vesiculosus, other species reported in previous works [7][8][58,64]. In addition, M. pyrifera and Undaria pinnatifida removed chromium and mercury from aqueous solutions. It was demonstrated that the carboxylic and amino groups are strongly involved in chromium binding, while amino and sulfhydryl are for mercury uptake, establishing that the interaction would be specific between metal and functional groups [5][6][62,63].
Brown alga Sargassum sp. was used to remove Pb and Cu from stormwater, resulting in biosorption capacities of 196.1 mg/g and 84.0 mg/g for Pb and Cu, respectively. The analysis of the functional groups of the algae using FTIR showed that the carboxyl was mainly responsible for biosorption [9][65].
The use of alginate from seaweed has also been validated. Barquilha et al. [19][74] used alginate from Sargassum sp. as a biosorbent for Ni and Cu ions from synthetic solutions and actual electroplating effluents. The experiments were carried out at pH 4.5. The maximum sorption capacity (qmax) of up to 1.147 mmol/g for Ni ions and 1.640 mmol/g for Cu ions. The biosorption of Ni and Cu from actual electroplating effluents with high concentrations of light metals becomes highly competitive, decreasing the amount of biosorbed Ni and Cu ions due to the effect of ionic strength.
On the other hand, a metal that shows considerable interest in its recovery is copper; despite being an essential element, when its concentration is high, it is potentially toxic. This generates the need to develop procedures for its elimination from natural environments. In that context, biosorption has been proven to be a very useful tool for its removal [20][41]. An advantage of the biosorption of copper with algal biomasses is the possibility of using dead biomass that handles mining effluents, which have unfavorable chemical characteristics for cell growth (acidity, ionic strength, and heavy metal content). Several algae can be cultivated in sea farms and are therefore considered a reliable source of supply. On the other hand, the biomass acts as an ion exchanger; therefore, the process is carried out quickly, and desorption of the metal is relatively easy. The use of marine algae, predominantly brown algae, to remove heavy metals from solutions through the mechanisms of biosorption and bioaccumulation was reviewed by Yadav et al. [21][75].
Lessonia nigrescens biomass was used to biosorb Cu in solution at pH 5 and contact time 120 min where the best data fit was the Langmuir isotherm model, obtaining a maximum biosorption capacity of 60.4 mg/g. The dead biomass of the brown algae L. nigrescens captures Cu ions in solution by surface interactions with different functional groups, such as carboxyl, hydroxyl, sulfonate and amide groups, but not amine groups [10][66].
3. Red Seaweeds
Cadmium was recovered from aqueous solutions with red alga Ceramium virgatum where experimental parameters that affect the biosorption process, such as pH, contact time, biomass dosage and temperature were studied. The C. virgatum monolayer biosorption capacity for Cd(II) was 39.7 mg/g ions [14][69]. Chaisuksan [12][67] studied the bioadsorption of pretreated red algae Gracilaria fisheri for cadmium and copper. The maximum uptake values were 0.63 and 0.72 mmol/g, respectively. Bioadsorption capacity increased as pH increased up to a plateau of four. Absorption rates for cadmium and copper were rapid with 90% biosorption completed in 30 min.
On the other hand, studies have removed Cu(II), Co(II) and Zn(II) ions from aqueous media using alginate extracted from the biomass of marine red algae Callithamnion corymbosum. The maximum biosorption capacities follow the order: Cu(II) (64.52 mg/g) > Zn(II) (37.04 mg/g) > Co(II) (18.79 mg/g), where the best biosorption conditions were at pH 4.4, biosorbent dose of 2.0 g/L and room temperature [15][70].
4. Green Seaweeds
The green algae Codium vermilara was used to remove copper, with an efficiency of about 85% under an algae dosage of 0.75 g/L, pH 5.3, contact time 70.5 min and copper concentration of 48.8 mg/L [16][71]. On the other hand, Chlorella vulgaris biosorbed copper with a recovery of 90.3% under pH 7, 105 min of contact time and 20 mg/L of initial copper concentration [17][72]. It was determined that the chemical groups involved in the green cell walls are preferably amine and carboxyl. The biosorption was extracellular due to the presence of copper on the cell surface.
