Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Dean Liu and Version 3 by Dean Liu.
The integration of nanofillers gives appropriate characteristics to the membrane distillation (MD) membranes by changing their chemical and physical properties, which significantly enhances energy efficiency without impacting the economic costs. Here, we provide a comprehensive overview of the state-of-the-art status, the opportunities, open challenges, and pitfalls of the emerging field of modified ENMs using different nanomaterials for desalination applications.
- electrospinning
- nanofibers
- nanomaterials
- modified membranes
1. Introduction
Water shortages have emerged as one of the century’s primary concerns because of industrialization, climate change, rising population, and modernization. Water is regarded as a basic requirement and source of sustenance for all living species on the planet; however, waste such as industrial effluents, heavy metals, volatile organic compounds, oil emulsions, and such impurities are major threats to marine life [1], which ultimately increases the demand for the development and improvement of water remediation technologies concerning recyclability and sustainability [2][3][4][5][6]. Amongst various water treatment techniques, membrane distillation (MD) is considered one of the leading-edge technologies for obtaining drinkable water from seawater, brine, or other wastewater resources [7]. The MD process can be operated with renewable energy sources such as solar, geothermal, or other waste-heat energy sources for low-cost water treatment [1][8]. However, many improvements and optimizations are required for their efficient production and deployment on a large scale [9][10][11][12][13][14][15].
In simple words, in an MD process, liquid–vapor separation occurs at the interface of a highly hydrophobic microporous membrane. A trans-membrane vapor pressure difference created due to the temperature difference across the membrane is the driving force in an MD process. Theoretically, the hydrophobic layer has the potential to reject non-volatile pollutants up to 100% that may be dissolved in the feed [16]. As a result, MD has received significant attention in water recovery from saline water as well as wastewater. However, when dealing with multiple effluents, including various types of low surface-tension components such as oils, grease, alcohols, organics, and surfactants, the membrane’s hydrophobicity becomes a concern, due to the affinity of various pollutants to the membrane surface. In this scenario, a pretreatment process is recommended before the MD process to remove all the organic pollutants [17].
2. Modification of ENMs with Functional Molecules and Their Effect on the MD Process
2.1. Effect of MOFs and Zeolites on ENMs and the MD Process
2.1.1. Aluminum Fumarate (AlFu) Addition
MOF-based ENMs are being widely explored in water treatment applications. MOFs possess unique characteristics such as high porosity and high specific surface area, and they can be functionalized with a variety of nanomaterials so that we can tune the MOF-based membranes as per the need of the MD process application. Leaching of nanomaterials from the polymer matrix is one of the problems faced during the water filtration process. This may lead to contamination of the product water with the nano-sized MOFs. The electrospinning of MOF-based polymer dope solutions followed by pyrolysis under controlled conditions has the potential to resolve the leaching issues [18], physically trapping them into the nanofibers, which ultimately resolves the issues of chemical compatibility as well the mechanical stability of the resultant membranes [18][19]. The current research trend on MD membrane fabrication reveals that the optimum incorporation of nano-additives significantly improves the performance of the MD process by improving the aforementioned MD membrane characteristics [20]. AlFu is a commercially available and widely used MOF for MD membrane fabrication. AlFu-based MOF is inexpensive, and it comes with multiple MD-friendly characteristics, including being hydrophobic, water stable, environmentally friendly, able to be sourced from water and simple aluminum salts, having a permanent three-dimensional highly porous structure and with a large-scale production capacity about 3600 kg/m3 per day. Therefore, an AlFu MOF is considered a promising agent for wastewater treatment and possibly other MD applications when utilized with ENMs [21]. AlFu can be incorporated into PVDF-HFP via electrospinning. The observed permeate flux was improved up to 114% during 46 h of continuous MD operation in comparison with neat PVDF-HFP ENMs. The thermal efficiency during the DCMD process was improved with the addition of AlFu MOFs in PVDF-HFP ENMs. The presence of AlFu MOF nanomaterials also enhanced the surface roughness, WCA, LEP, and thereby anti-wetting characteristics of the MD membrane [22]. Figure 3a shows a schematic diagram of the DCMD process while using AlFu-MOF-incorporated ENMs, revealing systematically ordered pores in MOFs with sizes between 0.6 and 0.7 nm [19], which offer an additional path for vapor transport and resulting in enhanced permeate flux [21]. Moreover, the presence of AlFu MOFs on the surface of ENMs in the form of protrusions enhances the surface roughness, which effectively increases the area for evaporation [23]. MAF-4 is a hydrophobic nanomaterial and interlayer that can be fabricated by seeding Zn (II) using the in-situ crystallization method. Because of the hydrophobic and anti-fouling characteristics of MAF-4, it has great potential to be utilized for MD. Figure 3b shows the step-by-step growth of MAF on a poly ether sulfone substrate via self-polymerization of Zn-seeded dopamine [24][25]. Only a few reports have been published on the application of MOF/MAF nanomaterials in MD membranes. In a recent report, 8% MAF-4 with PVDF could enhance water flux by 60% compared with neat PVDF membranes [26].2.1.2. Zeolitic Imidazolate Frameworks (ZIFs) Addition
The surface roughness and hydrophobicity of ENMs can be also enhanced by the addition of zeolites. A schematic representation of ZIF structures is given in Figure 1c. The zeolites belong to the MOF family that connects their imidazolate group with divalent metal cations [27]. ZIFs have high thermal resistance, chemical stability, and high porosity. The aforementioned characteristics of ZIFs can be utilized for applications including the fabrication of efficient MD membranes for water reclamation [28][29]. ZIFs contain organophilic imidazolate linkers, offering hydrophobic characteristics [30]. Additionally, Zeolitic imidazolate framework-71 (ZIF-71) offers enhanced hydrophobic properties due to the presence of methyl (–CH3) and chlorine (–Cl) entities in their chemical composition [27]. ZIF-71 NPs incorporated into PVDF-HFP significantly increased the surface roughness and hydrophobicity, yielding a permeate flux of 19.2 L m−2 h−1 with a salt rejection of >99.99%, which is 284% and 949% higher compared with the MD flux observed while using various pristine microporous membranes [29]. The PVDF-HFP ENM only had a WCA of 127.6°, while 0.75% ZIF-71 NP-incorporated PVDF-HFP ENMs showed increased hydrophobic properties with a WCA of 135°, and enhanced chemical and mechanical stability. The DCMD efficiency while using this nanostructured MD membrane was reported as 99.5% [19].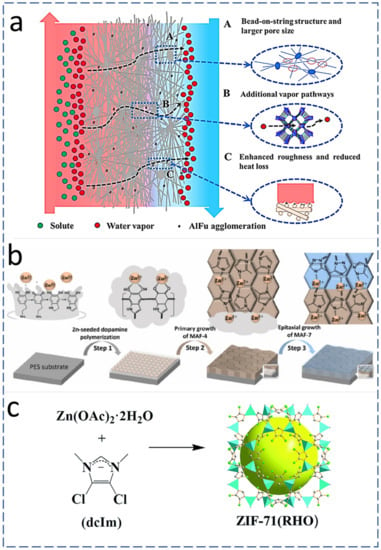
Figure 1. (a) Mechanism and effect of AlFu MOF on the DCMD performance [19] (Reprinted with the permission License No: 5487001092782 15 Febuary 2023, Elseviers). (b) MAF growth on PES polymer substrate and its conversion to other zeolitic species (MAF-7) [24] (Reprinted with the permission License No: 5490181160662 15 Febuary 2023, Elseviers). (c) Crystal structure of ZIF-71: Zn (polyhedral), Cl (green sphere), N (purple sphere), C (white sphere) [27] (Reprinted with the permission License No: 1324377-1 15 Febuary 2023, RSC Publishing).
2.2. Effect of SiO2, TiO2, and Zinc Oxide (ZnO) on ENMs and the MD Process
Nanomaterial additives such as SiO2, TiO2, and ZnO can be directly blended with the electrospinning dope solution, and this is a popular approach to obtain modified ENMs for the MD process [31][32]. ENMs incorporated with the aforementioned nanomaterials possess enhanced MD membrane characteristics such as WCA, LEP, mechanical strength, narrow pore size distribution, and controlled porosity [32][33][34][35]. It is shown that the MD membrane characteristics and calculated MD flux while using ENMs incorporated with SiO2, TiO2, and ZnO nanomaterials. In general, these NPs have been utilized for various applications such as biomedical, environmental, textile, and water treatment [36], and their details are discussed in the following section.2.2.1. SiO2 Addition
SiO2 NPs have been utilized for various applications because of their size range between 5 to 1000 nm, high adsorption capacity, high specific surface area, unique optical properties, low density, low toxicity level, and biocompatibility. As per the report, the LEP of ENMs with 1% SiO2 was 43% higher than the neat PVDF. It may be because the presence of additional nanomaterials on the membrane surface could reduce the pore size, enhancing the surface roughness and thereby the hydrophobicity and WCA [37]. Compared with neat hydrophobic PVDF ENMs with a WCA of 92.8°, PVDF ENMs with modified SiO2 NPs showed increased hydrophobicity with a WCA of 109°. Similar WCA has been reported for PVDF membranes with a 3–4% addition of SiO2 NPs [38]. The advantage of superhydrophobicity and the “lotus effect” is not limited to its anti-wetting characteristics, but also applies to the membrane’s anti-fouling properties and self-cleaning characteristics [39]. At a temperature of 60 °C, the MD water flux while using SiO2-incorporated PVDF ENM (19.4 L m−2 h−1) was 43% higher than that of neat PVDF ENM (13.6 L m−2 h−1). Water flux enhancements are also attributed to reduced pore blockage due to the reduced salt deposition or scaling propensity while employing SiO2 NP-modified ENMs during the MD process testing [40][41]. In one report, modified SiO2 NPs were incorporated into a PVDF polymeric matrix to obtain nanocomposite-based PVDF ENMs with a WCA as high as 147.8–161.2°, which was up to a 10.7% increase compared with the pristine PVDF [32]. Silanes are monomeric silicon compounds such as N-octadecyltrichlorosilane (ODTS), octadecyltrimethoxysilane (OTMS), chloromethyl-octadecyl silane (Cl-DMOS), etc., which are useful for improving the hydrophobic properties of ENMs by lowering their surface energy. The OTMS is an aliphatic long chain of carbon (CH3(CH2)17−) with (-Si-OCH3)3 as an anchor group [42], whereas Cl-DMOS and ODTS possess (-Si-Cl3)3 and (-Si-ClCH2)3, respectively, as the anchor groups [43][44]. These silanes have plenty of non-polar CH3 groups which impart hydrophobic characteristics. Apart from these non-polar groups, the strong electron-withdrawing atoms, viz., oxygen and chlorine in ODTS and Cl-DMOS, result in uneven electron distribution with minimum polarity at the respective sites, which ultimately increases the hydrophobic characteristics [45][46]. The superhydrophobic features may result in the “lotus effect” and thereby yield self-cleaning characteristics to the modified ENMs [39]. To achieve this effect, they must have a WCA close to 180° and a relatively lower sliding angle which may allow for the roll-off of water droplets. These properties would enhance the anti-fouling and anti-scaling characteristics of the membrane [36]. Several other studies have reported increased hydrophobic properties of MD membranes by the addition of SiO2 NPs and TiO2 NPs. These nanoparticles cause surface roughness, reduce the pore size, and impart respective functional groups to the surface to inhibit foulants or scalants during the MD process. The surface roughness results in increased WCA, which improves the hydrophobic characteristics of the ENM. This hydrophobic behavior creates air pockets that resist pore wetting and let the water droplets easily roll off over the membrane surface [4][36].2.2.2. TiO2 Addition
TiO2 is one of the key ingredients that has been utilized to fabricate composite membranes for different applications. TiO2 NPs show different behavior with regard to their potential affinity towards H2O or any waste effluents present in the water. TiO2 NPs are multifunctional due to their versatile characteristics such as large specific surface area and easily tunable chemical properties [47]. The hierarchical structure morphology can be generated by the deposition of TiO2 NPs using different tuning agents with desired surface properties. Additionally, TiO2 might offer reactive sites for covalent bonding between hydrolyzed silane coupling agents and hydroxyl groups (OH) available on the TiO2 surface, as shown in Figure 2a,b. The ENMs incorporated with TiO2 NP (38.71 L m−2 h−1) showed 5% and 45% enhanced initial flux compared with neat PVDF-HFP (36.78 L m−2 h−1) and commercial membranes (26.64 L m−2 h−1), respectively. The reason behind this is the optimum pore sizes and higher porosities of neat PVDF-HFP and ENMs incorporated with TiO2, compared to the commercial membranes. The PVDF-HFP ENMs showed a 5% more porous structure (82.6% porosity) compared with ENMs embedded with the organically modified SiO2 NPs (78.5% porosity). This slight decline in porosity was associated with the sprayed NPs on the surface of ENMs that somehow blocked the membrane pores. The PVDF-HFP exhibited a WCA of 142°, which is higher than the commercial membrane with a WCA of 118°. This is because of the rougher surface of the randomly deposited non-woven PVDF-HFP ENM. In one report, the authors compared the WCA of PVDF-HFP with dual layer membranes, which showed a 13°-enhanced WCA compared with one with modified TiO2, which had a WCA of 155° and low sliding angles of less than 20°. Moreover, the prepared superhydrophobic dual-layer membranes showed comparable LEP values compared to the commercial membranes [48]. Superhydrophobization can be performed by coating fluorosilane molecules onto TiO2 NPs by a low-temperature hydrothermal process. One of the fluorosilanization agents is 1H, 1H, 2H, 2H-perfluorododecyl trichlorosilane (FTCS), which offers sites of hydroxyl groups in TiO2 NPs for fluorosilanization and thereby achieves a hierarchical morphology. Hydrophobically modified TiO2 NPs can either blend with a polymeric dope solution to fabricate ENMs or disperse in a suitable medium and be coated onto ENMs by electrospraying [47]. The mechanism proposed in Figure 2a reveals the fluorosilanization of TiO2 on the surface of PVDF [47]. FTCS anchors hydrophilic trichlorosilane with chains of hydrophobic fluorinated carbon, which reveals the formation of trisilanols/hydrosilanes with hydrolyzation of the hydrophilic trichlorosilane head in water [49]. Further, Figure 2b shows the Si–O–Ti covalent bond formed because of the hydroxyl groups present in trisilanol molecules. These interactions between TiO2 and PVDF might offer robust, dense, hydrophobic surfaces which can be beneficial for the MD process [47].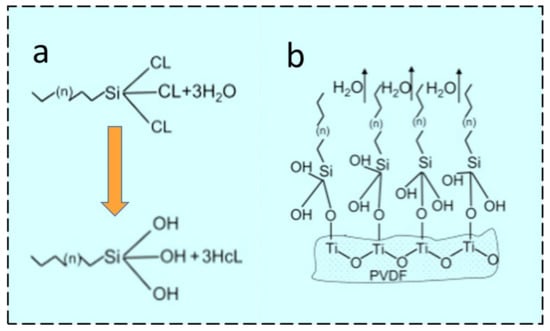
Figure 2. Scheme for the salinization of PVDF by (a) hydrolyzation using FTCS, or (b) condensation of trisilanols [47] (Reprinted with the permission License No: 5497580436300 14 February 2023, Elsevier).
2.2.3. ZnO Addition
ZnO NPs possess good thermal resistance, high surface-to-volume ratio, and anti-bacterial characteristics. In addition, these nanomaterials are considered environmentally friendlier and more economical than TiO2 and Al2O3 when utilized for surface modification of membranes. ZnO may offer anti-wetting, anti-fouling, and anti-scaling characteristics with reduced water sliding angles when treated with organo-silane molecules such as 1H, 1H, 2H, 2H-perfluorooctyltriethoxysilane (FAS). These features of ZnO with FAS offer a stable superhydrophobic surface, which directly indicates its potential to be utilized for MD [50]. PVDF-HFP with 25% ZnO NPs can be used to fabricate nanostructured ENMs. A stable DCMD flux rate was observed while using a low surface tension feed, with a salt rejection of 99.99% for up to 80 h of continuous operation. A slight compromise in water flux (up to 7% reduction) was observed when compared with neat PVDF-HFP ENMs. The WCA of ZnO NPs with PVDF-HFP ENMs was observed to be as high as 161°°, and the average pore size was 0.6 μm, which ultimately impacted the LEP of membranes and reached 187 kPa. This report confirms the potential of ZnO utilization for MD applications [50].2.3. Effect of CNT, GO, and AC on the MD Membranes and the MD Process
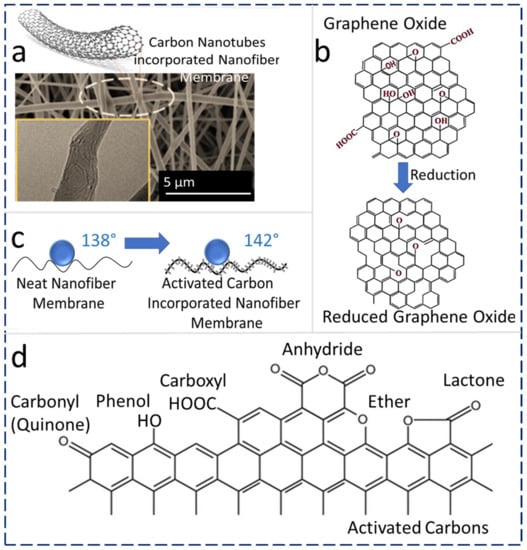
Carbon-based hydrophobic nanomaterials, viz., CNTs, graphene, and activated carbon (AC), are being widely utilized as additives in ENMs to fabricate MD membranes with desired characteristics [51][52][53].2.3.1. CNT Addition
Recently, researchers have optimized the concentration of CNTs in a PVDF-HFP polymeric matrix to obtain highly hydrophobic and robust MD membranes. A 0.5 wt% CNT–PVDF-HFP heat-pressed at 150 °C (CNT-150) yields a robust MD membrane with high DCMD permeate flux (16.5–18.5 L m−2 h−1), which is 42–50 % higher flux compared with commercially available membranes (11–13 L m−2 h−1) at similar operating conditions. ENMs generally show higher MD vapor flux than commercial membranes, which is primarily attributed to their unique porous structure and surface roughness. The porosity of untreated PVDF-HFP ENMs was 89%, while the membrane after heat-press (150 °C) treatment resulted in a decreased porosity (80%) because of the reduction in certain voids and pore size after heat treatment. Conversely, the stress at break and Young’s modulus of the treated ENM was increased due to the compaction of fibers after heat-press treatment. The WCA of PVDF-HFP ENMs was reduced from 135.9° to 123° because of the reduction in surface roughness as a result of the heat-press treatment and the melting of partial nanofibers. Thus, a controlled heat treatment yields robust ENMs with a slight reduction in the porosity and WCA. Additionally, 99.99% of inorganic non-volatile salt rejection can be achieved by using CNT-incorporated electrospun MD membranes during a DCMD process [51].2.3.2. GO Addition
Reduced graphene oxide (rGO) is hydrophobic, and it is considered a promising candidate for ENM-based nanocomposite MD membranes [54][55]. Fine and porous membranes based on GO exhibit a unique permeation pathway for water molecules. The rGO-ENMs have very good mechanical properties, chemical stability, flexibility, anti-fouling properties, and hydrophilicity [56]. The physicochemical comparison of GO and rGO is shown in Figure 3b. GO contains -O-, –OH, C=O, and –COOH groups, whereas rGO is a reduced form of GO with reduced hydrophilic characteristics, which is required for the MD process. The hydrophilicity of GO originates from the hydroxyl and epoxy groups in the basal plane, along with other functional groups such as carbonyl and carboxylic groups [57]. Octadecyl groups are substituted by GO groups upon functionalization [48], which results in an active surface area, higher hydrophobicity, higher surface contact angle, and lower surface energy. In a recent report, a commonly used three-stage method was used to fabricate superhydrophobic mixed-matrix PVDF-HFP ENMs that were modified using octadecylamine-reduced graphene oxide (ODA-rGO) and utilized for the MD process. The resultant PVDF-HFP ENMs with ODA-rGO showed a superhydrophobic nature with a WCA of 162°. The contact angles of the superhydrophobic nanofiber membranes containing 0.1 wt% and 0.5 wt% ODA-rGO were measured as 158° ± 1° and 156° ± 3°, respectively, which were about 14.5% and 13% higher than the contact angle of the pristine PVDF-HFP. Beads-on-string morphology was observed due to the agglomeration of ODA-rGO (0.5 wt%) during the elongation of the nanofibers. In addition, the fiber diameter could be tuned by changing the concentration of ODA-rGO in the dope solution [58]. Additionally, LiCl (0.005 wt%) was used to control the pore size of ENMs followed by a hot-pressing process. The average pore size of ENMs was reduced from 1.30 µm to 0.24 µm, and the LEP was improved from 30.4 kPa to 127.6 kPa in comparison with the pristine ENMs upon heat pressing. The tensile strength of hot-pressed rGO-ENMs was increased by about 26% compared with the neat ENMs [59].2.3.3. AC Addition
Nanostructured AC is widely utilized for water treatment applications because of the various available functional groups on its surface, which are beneficial for modifying the surface and its physical and chemical characteristics [60]. In a recent report, the effect of AC NP-based ENM’s hydrophobicity was studied, which revealed that the AC NPs form a hierarchical structure with micro-wrinkles or protrusions and results in increased surface roughness, ultimately increasing the surface hydrophobic properties. This is because of the result of weave-like surface roughness generated on the ENMs. This results in increased WCA for all samples incorporated with AC. Figure 3c shows a schematic illustration of an AC-anchored nanofiber structure [23]. Recently, dual-layer compositions with hydrophobic and hydrophilic layered membranes were prepared and utilized for the DCMD process. The selective hydrophobic PVDF-HFP ENMs were prepared with varying AC NP concentrations from 0 to 3.0 wt% along with other hydrophilic support layers [23]. Overall MD performance was improved with the composition of 1.5 wt% NPs, yielding a water vapor flux of 45.6 L/m2 h which is 9% higher compared with the MD flux while using commercially available PTFE membranes (41.8 L/m2 h) with no compromise in the salt rejection (99.99%) during the MD process. These resultant membranes have relatively wider pores, which contributed to an increase in the porosity by ~20% compared with the commercial PTFE membrane. The higher porosity of these fabricated ENMs is beneficial for reducing mass transfer resistance and leads to better permeation flux during the MD process. The ENMs exhibit relatively lower LEP values (1.21–1.36 bar) than the commercial PTFE membrane due to their larger pore size. However, the LEP values of these ENMs are quite comparable with other results obtained for MD membranes reported in the literature [61][62][63][64][65], which is considered to be adequate for DCMD applications. L-type and H-type are two kinds of ACs. H-type ACs are positively charged when treated under water or strong acids and thus possess a hydrophobic character. On the other hand, L-type ACs are neutralized by strong bases and possess hydrophilic properties [66]. Typical oxygen-containing surface functional groups in AC are depicted in Figure 3d, and these oxygen-containing surface functionalities are the routes for achieving desired surface properties during membrane fabrication [67].References
- Chiavazzo, E. Critical Aspects to Enable Viable Solar-Driven Evaporative Technologies for Water Treatment. Nat. Commun. 2022, 13, 5183.
- Abdulhamid, M.A.; Muzamil, K. Recent Progress on Electrospun Nanofibrous Polymer Membranes for Water and Air Purification: A Review. Chemosphere 2023, 310, 136886.
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A Review on Current Nanofiber Technologies: Electrospinning, Centrifugal Spinning, and Electro-Centrifugal Spinning. Macromol. Mater. Eng. 2022, 2022, 2200502.
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A Comprehensive Review on Preparation, Functionalization and Recent Applications of Nanofiber Membranes in Wastewater Treatment. J. Environ. Manag. 2022, 301, 113908.
- Santoro, S.; Avci, A.H.; Politano, A.; Curcio, E. The Advent of Thermoplasmonic Membrane Distillation. Chem. Soc. Rev. 2022, 51, 6087–6125.
- Tawalbeh, M.; al Mojjly, A.; Al-Othman, A.; Hilal, N. Membrane Separation as a Pre-Treatment Process for Oily Saline Water. Desalination 2018, 447, 182–202.
- Francis, L.; Ahmed, F.E.; Hilal, N. Electrospun Membranes for Membrane Distillation: The State of Play and Recent Advances. Desalination 2022, 526, 115511.
- Luo, Q.; Liu, P.; Fu, L.; Hu, Y.; Yang, L.; Wu, W.; Kong, X.Y.; Jiang, L.; Wen, L. Engineered Cellulose Nanofiber Membranes with Ultrathin Low-Dimensional Carbon Material Layers for Photothermal-Enhanced Osmotic Energy Conversion. ACS Appl. Mater. Interfaces 2022, 14, 13223–13230.
- Zhong, L.; Wang, Y.; Liu, D.; Zhu, Z.; Wang, W. Recent Advances in Membrane Distillation Using Electrospun Membranes: Advantages, Challenges, and Outlook. Environ. Sci. 2021, 7, 1002–1019.
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling Mitigation in Forward Osmosis and Membrane Distillation for Desalination. Desalination 2020, 480, 114338.
- Kim, S.; Heath, D.E.; Kentish, S.E. Robust and Superhydrophobic PTFE Membranes with Crosshatched Nanofibers for Membrane Distillation and Carbon Dioxide Stripping. Adv. Mater. Interfaces 2022, 9, 2200786.
- Hashaikeh, R.; Lalia, B.S.; Kochkodan, V.; Hilal, N. A Novel in Situ Membrane Cleaning Method Using Periodic Electrolysis. J. Memb. Sci. 2014, 471, 149–154.
- Alpatova, A.; Verbych, S.; Bryk, M.; Nigmatullin, R.; Hilal, N. Ultrafiltration of Water Containing Natural Organic Matter: Heavy Metal Removing in the Hybrid Complexation–Ultrafiltration Process. Sep. Purif. Technol. 2004, 40, 155–162.
- Hilal, N.; Ismail, A.F.; Wright, C.J. Membrane Fabrication; Routledge: London, UK, 2015; ISBN 9781138894099.
- Ismail, A.F.; Hilal, N.; Jaafar, J.; Wright, C.J. Nanofiber Membranes for Medical, Environmental, and Energy Applications; Routledge: London, UK, 2019; ISBN 9781032239859.
- Tan, G.; Xu, D.; Zhu, Z.; Zhang, X.; Li, J. Tailoring Pore Size and Interface of Superhydrophobic Nanofibrous Membrane for Robust Scaling Resistance and Flux Enhancement in Membrane Distillation. J. Memb. Sci. 2022, 658, 120751.
- Meng, L.; Mansouri, J.; Li, X.; Liang, J.; Huang, M.; Lv, Y.; Wang, Z.; Chen, V. Omniphobic Membrane via Bioinspired Silicification for the Treatment of RO Concentrate by Membrane Distillation. J. Memb. Sci. 2022, 647, 120267.
- Peng, R.; Zhang, S.; Yao, Y.; Wang, J.; Zhu, X.; Jiang, R.; Zhang, J.; Zhang, W.; Wang, C. MOFs Meet Electrospinning: New Opportunities for Water Treatment. Chem. Eng. J. 2023, 453, 139669.
- Wu, X.Q.; Mirza, N.R.; Huang, Z.; Zhang, J.; Zheng, Y.M.; Xiang, J.; Xie, Z. Enhanced Desalination Performance of Aluminium Fumarate MOF-Incorporated Electrospun Nanofiber Membrane with Bead-on-String Structure for Membrane Distillation. Desalination 2021, 520, 115338.
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal-Organic Frameworks Supported on Nanofiber for Desalination by Direct Contact Membrane Distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260.
- Cheng, D.; Zhao, L.; Li, N.; Smith, S.J.D.; Wu, D.; Zhang, J.; Ng, D.; Wu, C.; Martinez, M.R.; Batten, M.P.; et al. Aluminum Fumarate MOF/PVDF Hollow Fiber Membrane for Enhancement of Water Flux and Thermal Efficiency in Direct Contact Membrane Distillation. J. Memb. Sci. 2019, 588, 117204.
- Schneider, R.; Facure, M.H.M.; Chagas, P.A.M.; Andre, R.S.; dos Santos, D.M.; Correa, D.S. Tailoring the Surface Properties of Micro/Nanofibers Using 0D, 1D, 2D, and 3D Nanostructures: A Review on Post-Modification Methods. Adv. Mater. Interfaces 2021, 8, 2100430.
- Zhao, L.; Wu, C.; Lu, X.; Ng, D.; Truong, Y.B.; Xie, Z. Activated Carbon Enhanced Hydrophobic/Hydrophilic Dual-Layer Nanofiber Composite Membranes for High-Performance Direct Contact Membrane Distillation. Desalination 2018, 446, 59–69.
- Wang, Z.; Qi, J.; Lu, X.; Jiang, H.; Wang, P.; He, M.; Ma, J. Epitaxially grown MOF membranes with photocatalytic bactericidal activity for biofouling mitigation in desalination. J. Membr. Sci. 2021, 630, 119327.
- Ruan, X.; Zhang, X.; Liao, X.; Jiang, X.; Dai, Y.; Yan, X.; He, G. Enhancing Mechanical Stability and Uniformity of 2-D Continuous ZIF-8 Membranes by Zn(II)-Doped Polydopamine Modification. J. Memb. Sci. 2017, 541, 101–107.
- Wu, R.; Tan, Y.; Meng, F.; Zhang, Y.; Huang, Y.X. PVDF/MAF-4 Composite Membrane for High Flux and Scaling-Resistant Membrane Distillation. Desalination 2022, 540, 116013.
- Wang, Y.-H.; Shi, Q.; Xu, H.; Dong, J.-X. The Synthesis and Tribological Properties of Small-and Large-Sized Crystals of Zeolitic Imidazolate Framework-71. RSC Adv. 2016, 6, 18052–18059.
- Xu, S.; Ren, L.F.; Zhou, Q.; Bai, H.; Li, J.; Shao, J. Facile ZIF-8 Functionalized Hierarchical Micronanofiber Membrane for High-Efficiency Separation of Water-in-Oil Emulsions. J. Appl. Polym. Sci. 2018, 135, 46462.
- Huang, M.; Song, J.; Deng, Q.; Mu, T.; Li, J. Novel Electrospun ZIF/PcH Nanofibrous Membranes for Enhanced Performance of Membrane Distillation for Salty and Dyeing Wastewater Treatment. Desalination 2022, 527, 115563.
- Huang, K.; Li, Q.; Liu, G.; Shen, J.; Guan, K.; Jin, W. A ZIF-71 Hollow Fiber Membrane Fabricated by Contra-Diffusion. ACS Appl. Mater. Interfaces 2015, 7, 16157–16160.
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of Polyvinylidene Fluoride (PVDF) Nanofiber Membranes by Electro-Spinning for Direct Contact Membrane Distillation. J. Memb. Sci. 2013, 425–426, 30–39.
- Wang, S.; Li, Y.; Fei, X.; Sun, M.; Zhang, C.; Li, Y.; Yang, Q.; Hong, X. Preparation of a Durable Superhydrophobic Membrane by Electrospinning Poly (Vinylidene Fluoride) (PVDF) Mixed with Epoxy–Siloxane Modified SiO2 Nanoparticles: A Possible Route to Superhydrophobic Surfaces with Low Water Sliding Angle and High Water Contact Angle. J. Colloid Interface Sci. 2011, 359, 380–388.
- Kang, D.H.; Kang, H.W. Surface Energy Characteristics of Zeolite Embedded PVDF Nanofiber Films with Electrospinning Process. Appl. Surf. Sci. 2016, 387, 82–88.
- Ren, L.F.; Xia, F.; Chen, V.; Shao, J.; Chen, R.; He, Y. TiO2-FTCS Modified Superhydrophobic PVDF Electrospun Nanofibrous Membrane for Desalination by Direct Contact Membrane Distillation. Desalination 2017, 423, 3–11.
- Nthunya, L.N.; Gutierrez, L.; Verliefde, A.R.; Mhlanga, S.D. Enhanced Flux in Direct Contact Membrane Distillation Using Superhydrophobic PVDF Nanofibre Membranes Embedded with Organically Modified SiO2 Nanoparticles. J. Chem. Technol. Biotechnol. 2019, 94, 2826–2837.
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Nxumalo, E.N.; Verliefde, A.R.; Mamba, B.B.; Mhlanga, S.D. A Review of Nanoparticle-Enhanced Membrane Distillation Membranes: Membrane Synthesis and Applications in Water Treatment. J. Chem. Technol. Biotechnol. 2019, 94, 2757–2771.
- Kanduč, M.; Netz, R.R. From Hydration Repulsion to Dry Adhesion between Asymmetric Hydrophilic and Hydrophobic Surfaces. Proc. Natl. Acad. Sci. USA 2015, 112, 12338–12343.
- Dong, Z.-Q.; Ma, X.-H.; Xu, Z.-L.; Gu, Z.-Y. Superhydrophobic Modification of PVDF–SiO2 Electrospun Nanofiber Membranes for Vacuum Membrane Distillation. RSC Adv. 2015, 5, 67962–67970.
- Zhang, M.; Feng, S.; Wang, L.; Zheng, Y. Lotus Effect in Wetting and Self-Cleaning. Biotribology 2016, 5, 31–43.
- Bonyadi, S.; Chung, T.S. Flux Enhancement in Membrane Distillation by Fabrication of Dual Layer Hydrophilic–Hydrophobic Hollow Fiber Membranes. J. Memb. Sci. 2007, 306, 134–146.
- Kyoungjin An, A.; Lee, E.-J.; Guo, J.; Jeong, S.; Lee, J.-G.; Ghaffour, N. Enhanced Vapor Transport in Membrane Distillation via Functionalized Carbon Nanotubes Anchored into Electrospun Nanofibres OPEN; Nature Publishing Group: Berlin, Germany, 2017.
- Iwasa, J.; Kumazawa, K.; Aoyama, K.; Suzuki, H.; Norimoto, S.; Shimoaka, T.; Hasegawa, T. In Situ Observation of a Self-Assembled Monolayer Formation of Octadecyltrimethoxysilane on a Silicon Oxide Surface Using a High-Speed Atomic Force Microscope. J. Phys. Chem. C 2016, 120, 2807–2813.
- Khumalo, N.; Nthunya, L.; Derese, S.; Motsa, M.; Verliefde, A.; Kuvarega, A.; Mamba, B.B.; Mhlanga, S.; Dlamini, D.S. Water Recovery from Hydrolysed Human Urine Samples via Direct Contact Membrane Distillation Using PVDF/PTFE Membrane. Sep. Purif. Technol. 2019, 211, 610–617.
- Wang, Y.; Lieberman, M. Growth of Ultrasmooth Octadecyltrichlorosilane Self-Assembled Monolayers on SiO2. Langmuir 2003, 19, 1159–1167.
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18.
- Eykens, L.; de Sitter, K.; Dotremont, C.; Pinoy, L.; van der Bruggen, B. Membrane Synthesis for Membrane Distillation: A Review. Sep. Purif. Technol. 2017, 182, 36–51.
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic Modification of TiO2 Nanocomposite PVDF Membranes for Applications in Membrane Distillation. J. Memb. Sci. 2012, 415–416, 850–863.
- HMTShirazi, R.; Mohammadi, T.; Asadi, A.A.; Tofighy, M.A. Electrospun Nanofiber Affinity Membranes for Water Treatment Applications: A Review. J. Water Process Eng. 2022, 47, 102795.
- Milanesi, F.; Cappelletti, G.; Annunziata, R.; Bianchi, C.L.; Meroni, D.; Ardizzone, S. Siloxane-TiO2 Hybrid Nanocomposites. the Structure of the Hydrophobic Layer. J. Phys. Chem. C 2010, 114, 8287–8293.
- Deka, B.J.; Guo, J.; An, A.K. Robust Dual-Layered Omniphobic Electrospun Membrane with Anti-Wetting and Anti-Scaling Functionalised for Membrane Distillation Application. J. Memb. Sci. 2021, 624, 119089.
- Yan, K.K.; Jiao, L.; Lin, S.; Ji, X.; Lu, Y.; Zhang, L. Superhydrophobic Electrospun Nanofiber Membrane Coated by Carbon Nanotubes Network for Membrane Distillation. Desalination 2018, 437, 26–33.
- Leaper, S.; Abdel-Karim, A.; Gorgojo, P. The Use of Carbon Nanomaterials in Membrane Distillation Membranes: A Review. Front. Chem. Sci. Eng. 2021, 15, 755–774.
- Song, J.; Deng, Q.; Huang, M.; Kong, Z. Carbon Nanotube Enhanced Membrane Distillation for Salty and Dyeing Wastewater Treatment by Electrospinning Technology. Environ. Res. 2022, 204, 111892.
- You, Y.; Sahajwalla, V.; Yoshimura, M.; Joshi, R.K. Graphene and Graphene Oxide for Desalination. Nanoscale 2015, 8, 117–119.
- Ma, W.; Yang, L.; Chen, T.; Ye, Z.; Tufenkji, N.; Rahaman, M.S. Engineering Polymer Forest on Membranes: Tuning Density, Thickness, and Architecture for Biofouling Control. ACS Appl. Polym. Mater. 2020, 2, 4592–4603.
- Pandey, R.P.; Kallem, P.; Hegab, H.M.; Rasheed, P.A.; Banat, F.; Hasan, S.W. Cross-Linked Laminar Graphene Oxide Membranes for Wastewater Treatment and Desalination: A Review. J. Environ. Manag. 2022, 317, 115367.
- Lin, Z.; Liu, Y.; Wong, C.P. Facile Fabrication of Superhydrophobic Octadecylamine-Functionalized Graphite Oxide Film. Langmuir 2010, 26, 16110–16114.
- Bae, J.; Baek, I.; Choi, H. Mechanically Enhanced PES Electrospun Nanofiber Membranes (ENMs) for Microfiltration: The Effects of ENM Properties on Membrane Performance. Water Res. 2016, 105, 406–412.
- Fouladivanda, M.; Karimi-Sabet, J.; Abbasi, F.; Moosavian, M.A. Step-by-Step Improvement of Mixed-Matrix Nanofiber Membrane with Functionalized Graphene Oxide for Desalination via Air-Gap Membrane Distillation. Sep. Purif. Technol. 2021, 256, 117809.
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated Carbon Modifications to Enhance Its Water Treatment Applications. An Overview. J. Hazard. Mater. 2011, 187, 1–23.
- Liao, Y.; Wang, R.; Fane, A.G. Engineering Superhydrophobic Surface on Poly(Vinylidene Fluoride) Nanofiber Membranes for Direct Contact Membrane Distillation. J. Memb. Sci. 2013, 440, 77–87.
- Li, X.; Wang, C.; Yang, Y.; Wang, X.; Zhu, M.; Hsiao, B.S. Dual-Biomimetic Superhydrophobic Electrospun Polystyrene Nanofibrous Membranes for Membrane Distillation. ACS Appl. Mater. Interfaces 2014, 6, 2423–2430.
- Liao, Y.; Loh, C.H.; Wang, R.; Fane, A.G. Electrospun Superhydrophobic Membranes with Unique Structures for Membrane Distillation. ACS Appl. Mater. Interfaces 2014, 6, 16035–16048.
- Liao, Y.; Wang, R.; Fane, A.G. Fabrication of Bioinspired Composite Nanofiber Membranes with Robust Superhydrophobicity for Direct Contact Membrane Distillation. Environ. Sci. Technol. 2014, 48, 6335–6341.
- Li, X.; Deng, L.; Yu, X.; Wang, M.; Wang, X.; García-Payo, C.; Khayet, M. A Novel Profiled Core–Shell Nanofibrous Membrane for Wastewater Treatment by Direct Contact Membrane Distillation. J. Mater. Chem. A Mater. 2016, 4, 14453–14463.
- Ahmad, A.; Azam, T. Bottled and Packaged Water; Water Purification Technologies: Woodridge, IL, USA, 2019; pp. 83–120.
- Shiraishi, S. Activated Carbons. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 1–7.
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostructure Chem. 2018, 8, 123–137.
More