You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Seid Mahdi Jafari.
Cannabidiol (CBD), one of the most promising constituents isolated from
Cannabis sativa
, exhibits diverse pharmacological actions. The applications of CBD are restricted mainly due to its poor oral bioavailability. Therefore, researchers are focusing on the development of novel strategies for the effective delivery of CBD with improved oral bioavailability.
- Cannabis sativa
- cannabidiol
- molecular mechanism
1. Introduction
The plant cannabis or Cannabis sativa L. is among the plants with the longest histories of cultivation for its medicinal properties. It produces many phytochemicals such as cannabinoids, terpenes, and flavonoids, and is a major source of cannabinoids including cannabidiol (CBD), cannabinol (CBN), cannabigerol (CBG), tetrahydrocannabinol (THC), and cannabichromene (CBC) [1]. Initial use of cannabis dates back 5000 years in China [2]. Cannabinoids exhibit numerous potential pharmacological activities against nausea, epilepsy, inflammation, depression, and other clinically relevant effects [3]. Recently, there has been an increasing global acceptance of cannabinoids, due to the decoding of its genome description and molecular genetics. Moreover, the determination of the possible heteromeric complex formations, along with crystal structure determination of the CB1R and CB2R receptors, has led to the discovery and subsequent understanding of the human endocannabinoid system (ECS). The ECS refers to the cannabinoid receptors CB1, CB2, CB3, and other associated compounds that are activated by cannabinoids derivatives, which may include cannabinoids, endocannabinoids, other related compounds, along with their metabolic enzymes. Furthermore, detailed study involving cellular, molecular, biochemical and behavioral responses have revealed that CNR1 and CNR2 genes are mapped by the human chromosome 6 and 1, respectively, and are encoded by the use of cannabis and cannabinoids [4].
CBD usually contains a tetrahydro-biphenyl skeleton. A bicyclic core represents an adduct formed by the monoterpene, p-cymene, and the alkylresorcinol derivative olivetol [5]. Moreover, via an acid-catalyzed reaction, it can be converted into the tricyclic dibenzopyrane, Δ9-THC (THC) [6]. Although CBD and THC are predominantly found in plants of the cannabis genus, they just form a part of the bigger terpenyl-alkylresorcinol chemical subspace. Cannabinoids’ natural expression undergoes tremendous changes during its growth and usually consists of four major classes of molecules, each characterized by distinct skeletons [6]. All classes contain an olivetol core in which a hydroxyl group is replaced by an alkyl chain (Figure 1).
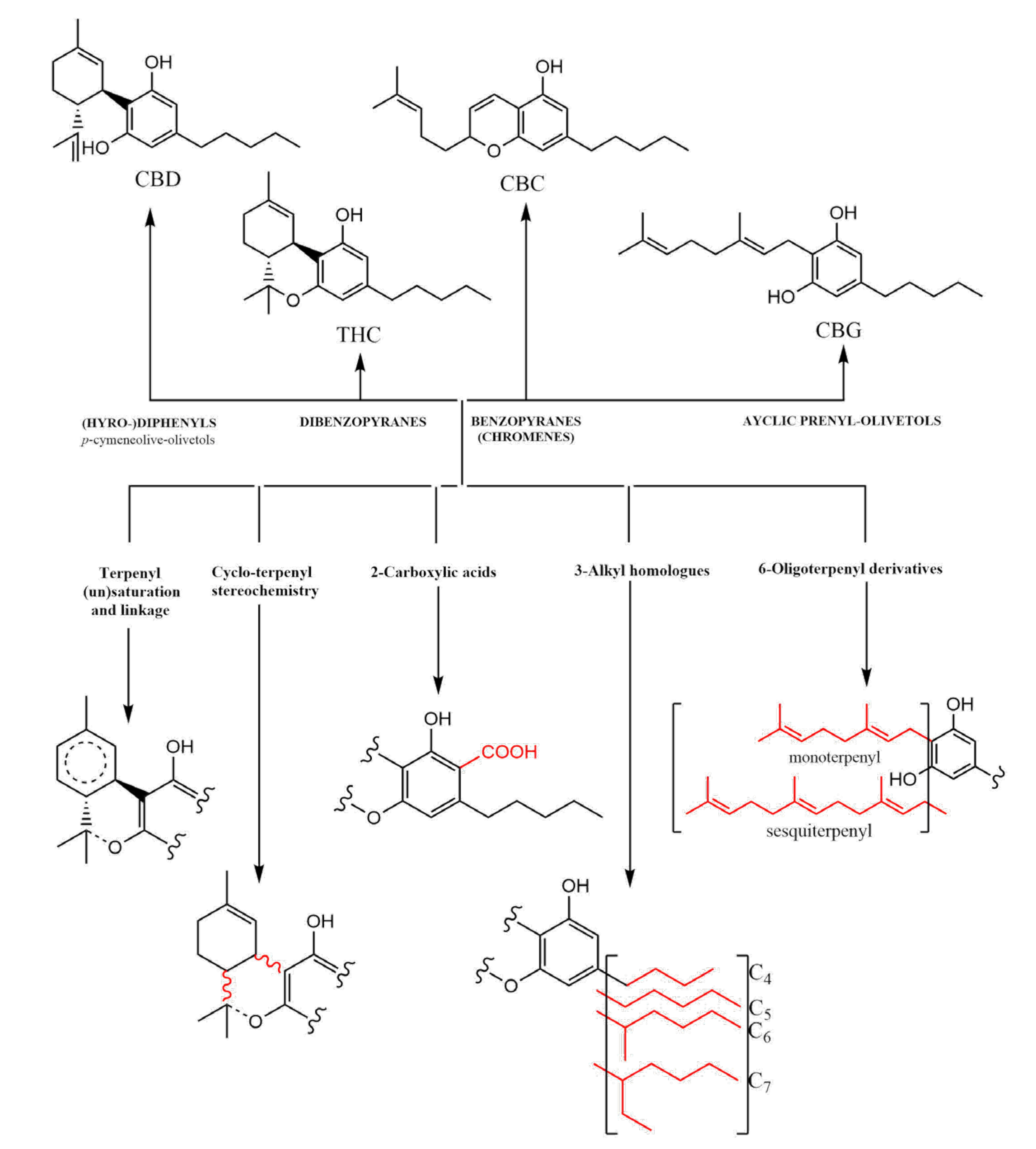
Figure 1. Major classification of cannabinoids (dibenzopyranes, benzopyranes, and acyclic prenyl-olivetols) based on the tetrahydro-diphenyl skeleton.
In addition to the bicyclic tetrahydro-diphenyl and tricyclic dibenzopyrane classes, benzopyranes (prototype: cannabichromene [CBC]) and acyclic prenyl-olivetols (prototype: cannabigerol [CBG]) are the other key classes of cannabinoids. These can undergo further structural modifications, expanding into five major dimensions: (i) variation of terpenyl saturation and linkage; (ii) terpenyl stereochemistry; (iii) presence vs. absence of C-2 carboxylation (“acids”); (iv) variation of length and branching of the olivetol alkyl chain (e.g., homologous cannabinoids) [7]; and (v) presence and variation of the monoterpene vs. sesquiterpene moieties. These collectively give some natural cannabinoid metabolites, as they are only one family among several other phytochemical classes in cannabis plants.
2. Cannabidiol (CBD): An Overview
Cannabidiol (CBD) is therapeutically used in a number of diseases such as Alzheimer’s disease/dementia, glaucoma, traumatic brain injury/spinal cord injury, chemotherapy-induced nausea/vomiting, appetite and weight loss, chronic pain, cancer, Huntington’s disease, Parkinson’s disease, dystonia, addiction, anxiety, depression, irritable bowel syndrome, epilepsy, spasticity of multiple sclerosis, Tourette syndrome, amyotrophic lateral sclerosis, sleep disorders, posttraumatic stress disorder, and schizophrenia.
2.1. Bioavailability and Safety of CBD and Associated Derivatives
The bioavailability of CBD mainly depends upon its mode and route of administration [24,25][8][9]. CBD is mostly formulated as a solution, either in oil or alcohol form, which is then processed as either soft gelatin capsules, oral solution, oromucosal spray or sublingual drops. There is huge uncertainty concerning the bioavailability of CBD administered through different routes; CBD and THC administered via oral and oromucosal routes exhibit high inter/intra-individual variability. Overall, the oral bioavailability of CBD is estimated to be around 6–9%, with tmax hovering between 1–4 h. A gelatin capsule containing 20 mg CBD exhibited a half-life = 1.4–10.9 h, and Cmax = 2.4 ng/mL. Administration of CBD along with a high calorie/fat meal is shown to increase its oral bioavailability and is one of the potential techniques used to increase its bioavailability. This has been tested in several individuals, and subjects in fed state exhibited 4- and 14-fold increase in bioavailability and Cmax, respectively [26,27][10][11].
2.2. Extraction
Extraction in the hemp industry is mainly performed in two ways: (a) extraction from trichomes, and (b) extraction from seeds. Extraction from trichomes is mainly done to obtain medicinal or recreational products. It mostly yields cannabinoids and terpenes. By contrast, in seed extraction, fatty acids are extracted, which finds use as biodiesel and in the cosmetics and food industries. Cannabinoids and terpenes are extracted from female flower buds just before pollination using polar solvents, as the concentration of cannabinoids are at peak levels just before flowering. The most employed methods for extraction include organic solvent extraction (OSE), supercritical fluid extraction (SFE), and Soxhlet apparatus. Mild comminution can be done for a better yield. Care should be taken to avoid excessive grinding as it may lead to the co-extraction of undesirable products [28,29][12][13]. Usually, along with the main components THC and CBD, waxes and pigments are also co-extracted.
A number of studies employing Soxhlet apparatus have extracted cannabinoids [1]. In the OSE method, the plant biomass is left immersed in an organic solvent for a defined period, for an effective mass transfer, whereas the SFE method uses the safe and capable solvents in their critical state to efficiently extract CBD. SFE enables selective extractions by increasing the temperature and pressure to critical state, where no liquid can be further liquefied at higher pressure, further increasing the liquid density [14]. Various solvents such as ethene, water, methanol, carbon dioxide, nitrous oxide, sulfur hexafluoride, n-butene, and n-pentane are commonly employed in SFE [14].
2.3. Biological Effects of CBD
2.3.1. Roles of CBD in the Immune System
CBD has been found to interact with the immune system in several ways, both positive and negative. The immune system is responsible for protecting the body from harmful pathogens and other foreign invaders. One of the most promising areas of research on CBD and the immune system is its potential to reduce inflammation. Inflammation is a natural response of the immune system to injury or infection, but chronic inflammation can lead to a host of diseases, including cancer, heart disease, and autoimmune disorders [30,31][15][16].
CBD has been found to have anti-inflammatory properties, mostly proved in several in vitro and in vivo disease models of inflammation. CBD has been proved to interact in inflammation processes through different mechanisms, both by innate and adaptive responses: inhibiting the production of inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α and IL-17A); reducing the production of nitric oxide (NO) or myeloperoxidase (MPO) pro- from innate cells; involving suppression of inducible nitric oxide synthase (iNOS); mediating apoptosis in lymphocytes contributing to immune suppressive mechanism; and decreasing microglial cell activation, among others [30,31][15][16]. On the other hand, it is important to highlight the relationship between inflammation and redox imbalance. In this regard, CBD has been shown to affect this balance by modifying the enzymatic activity of anti- and pro-oxidants, as well as the occurrence of other processes such as transition metal ions chelation, micronutrients supporting antioxidant activity, or oxidative modifications of lipids, proteins, or DNA [4].
CBD has also been studied in the context of autoimmune disorders. Some research suggests that CBD may be able to modulate the immune response in these conditions, potentially reducing symptoms and improving quality of life. The role of CBD in autoimmune diseases acts through microglial activation, T-cell proliferation, and suppressing the actions of proapoptotic proteins [30,31][15][16] However, it is important to note that the research on CBD and the immune system is still in its early stages, and more research is needed to fully understand its effects on physiological immune response.
2.3.2. Roles of CBD in the Nervous System
Cannabidiol (CBD) has gained attention in recent years for its potential therapeutic benefits, particularly in the nervous system. CBD interacts with the body’s endocannabinoid system (ECS), which plays a crucial role in regulating various physiological processes including sleep, appetite, pain, immune function and nervous system derived pathologies. CBD interacts with the ECS by binding to non-psychoactive receptors such as CB1 and CB2 [31][16] Pre-clinical research shows that CBD has ability to reduce anxiety or depression and improve mood, not acting through the CB1 receptor but interacting with other targets involved in neurologic disorders. However, the therapeutic effectiveness of CBD in anxiety disorders needs to be claimed in further clinical trials [31][16].
CBD also exhibits analgesic and neuroprotective effects, protecting neurons from damage and death caused by inflammation and oxidative stress. This could be beneficial in the treatment of neurological disorders such as Alzheimer’s and Parkinson’s disease or multiple sclerosis [31][16]. The mechanisms responsible for the potential analgesic and neuroprotective effects of CBD in nervous system disorders are not entirely understood [32][17]. For example, in the case of analgesic effects, it is known that the anxiolytic properties of CBD may have an influence on the occurrence of pain. The mechanisms that CBD uses to protect neurons are diverse. Some of them are related to the ECS (CB1 and CB2) system but many others use non-CBD-mediated mechanisms: serotonin receptors by 5HT1A facilitation, oxidative stress, mitochondrial dysfunction, peroxisome proliferator-activated receptor gamma (PPAR-γ), inflammatory changes (modulating pro-inflammatory cytokines and elevation of brain-derived neurotrophic factor [BDNF] levels), upregulation of the mRNA levels for Cu–Zn superoxide dismutase (SOD), preventing apoptotic signaling via a restoration of Ca2+ homeostasis, and β-amyloid (Aβ) aggregation and stimulation by upregulating the Wnt/b-catenin pathway [31,32,33][16][17][18].
CBD may also play a role in the treatment of epilepsy. Studies have shown that CBD can reduce the frequency and severity of seizures in individuals with epilepsy. This led to the FDA’s approval of the first CBD-based medication, Epidiolex, for the treatment of seizures associated with certain rare forms of epilepsy [34][19]. It is hypothesized that these beneficial properties could be linked to a “multi-target drug” profile involving some of the mechanisms explained before and more. Nevertheless, clinical trials are still ongoing to support this evidence [35][20].
2.3.3. Roles of CBD in the Cardiovascular System
CBD has been studied for its potential medicinal properties, including its beneficial effects on the cardiovascular system. CBD can be involved in various cardiovascular diseases (CVD) including myocardial ischaemia/reperfusion injury, heart failure, atherosclerosis, hypertension, and arrhythmias [31,36][16][21].
As previously stated, CBD has been shown to have anti-inflammatory and antioxidant effects, which may help to protect the cardiovascular system. Studies in animals and cell cultures have found that CBD can generally reduce the formation of blood clots, lower blood pressure, and improve blood flow, and more specifically, enhance vasorelaxant responses, reduce the vascular hyperpermeability, and protect against ischaemia-reperfusion damage and cardiomyopathy associated with diabetes. Additionally, CBD has been shown to reduce the risk of heart attack and stroke by reducing the activity of certain enzymes that contribute to the formation of plaques in the arteries and influencing the survival, death, and migration of white blood cells [37][22].
These effects are usually due to CBD’s direct interactions with the ECS (CB receptors) through agonistic and antagonistic action, by promoting or inhibiting the release of neurotransmitters, respectively [31][16]. However, CBD can also act indirectly by modulating the concentration of active compounds, i.e., inhibiting adenosine, thymidine, glutamate, serotonin, dopamine, and noradrenaline uptake [38][23]. Additionally, CBD has been also proposed to have a vascular effect through the release of vasorelaxant mediators as nitric oxide (NO) and/or actions mediated by vascular enzymes as well as cycloxygenase isoenzymes (COX) and superoxide dismutase (SOD) [31,38][16][23].
Some studies have found that CBD can lower the risk of atherosclerosis and have a beneficial effect on the cardiovascular system by reducing the risk of obesity-related CVD. While more research is needed to fully understand the mechanisms by which CBD may benefit the cardiovascular system, it should be noted that, although this compound has potential, it is not a substitute for traditional medical treatment.
3. Nanocarriers: A Potential Platform for Targeted Delivery of CBD
Nanocarriers have been used as a potential platform for the targeted delivery of various phytocompounds including CBD. Nanodelivery systems help in improving the stability of phytocompounds, enhance their absorption, protect them from early enzymatic deprivation or metabolism within the body, and extend their circulation time, thus limiting the various adverse effects [75][24]. The modified nanocarriers improve the solubility and permeability, and assist in the sustained delivery of CBD to the targeted diseased sites, thus improving the bioavailability. Various studies reporting on the effective delivery of CBD through these nanocarrier systems briefly shown in Figure 2.
Figure 2. Various nanocarriers improve the targetability and therapeutic efficacy of CBD.
3.1. Lipid-Based Nanocarriers for CBD
Various lipid-based nanocarriers have been reported for the effective and site-specific delivery of CBD, including nanoliposomes, nanoemulsions, nanostructured lipid carriers (NLCs), and solid lipid nanoparticles (SLNs). The advent of CBD-loaded in lipid-based nanocarriers presented greater therapeutic effects against different diseases and disorders [9,11][25][26].3.1.1. Pro-Nanolipospheres (PNLs)
PNLs are a group of lipid-based delivery systems that are homogenous and isotropic combinations of a hydrophobic compound with a mixture of lipids, co-solvents, and surfactants. The term “pre-concentrates” is related to anhydrous liquid mixtures that after combination with an aqueous phase can form oil-in-water nanoemulsions with droplet size of 200 nm or less [76][27]. For the preparation of PNLs, amphiphilic co-solvent and emulsifying excipients are first completely dissolved, and then a mixture of lipids and surfactants are added and stirred to form a homogenous solution. Finally, the pre-concentrate containing CBD is added and stirred to finally form an oil-in-water nano-dispersion [10][28]. The hydrophobic bioactive such as CBD can be incorporated into the lipid core of obtained nanoparticles. Cherniakov et al. [10][28] developed advanced PNLs containing natural absorption enhancers such as curcumin, piperine, and resveratrol, which can hinder certain metabolism processes. Their results indicated that the bioavailability of incorporated CBD in advanced PNLs was enhanced up to six-fold in comparison to pure CBD [10][28]. Another advantage is the solubility of CBD in lipid excipients, which provides greater and constant bioavailability [76][27].3.1.2. Nanoliposomes
Nanoliposomes are liposomes with nano size which are suitable for the incorporation of bioactive compounds with different hydrophobicity. Nanoliposomes have a hydrophilic core surrounded by one or more phospholipid bilayers. For the preparation of nanoliposomes, dipole substances such as phospholipids are mixed with water using different mechanical (e.g., high-pressure homogenization, ultrasonication, and microfluidization), and non-mechanical (e.g., reversed-phase evaporation) methods to form nano-sized vesicles [77,78][29][30]. A nanoliposome was formed by means of a sunflower lecithin (phosphatidyl choline) base to enhance the bioavailability of CBD. Nanoliposomal CBD was around 100 nm and contained 10–20 mg/mL CBD which showed high stability at both refrigerated and room temperatures at pH 5–9 for three months [79][31]. In another study, liposomal CBD was formulated using hydrogenated soy phosphatidylcholine with a median particle size of 5.6 μm, containing 50 mg/g of a synthetic CBD, to improve its bioavailability for use in pain management in dogs [80][32]. Nanoliposomes have advantages such as the possibility of large-scale production and high targetability, as well as delivery of amphiphilic molecules but rapid release [78][30].3.1.3. Transferosomes
Transferosomes are a class of liposomes with high elasticity, composed of phospholipids (such as phosphatidylcholine) and different surfactants (e.g., Tween 80, sodium cholate, deoxycholate, dipotassium glycyrrhizinate, and Span 80). The surfactants are responsible for the deformability of the obtained vesicles. The formulation of transferosomes may contain a total lipid concentration of ≤10% in the final aqueous lipid suspension and also some ethanol, usually <10% [81][33]. Transferosomes comprising cholesterol, soya lecithin, and Polysorbate 80 were produced through thin film evaporation. The transferosomal dispersion containing CBD showed an average diameter of 102–130 nm. It was observed that the stability of CBD was improved by up to six months at room temperature after encapsulation in transferosomes [82][34]. Compared to other liposomes, transferosomes have the advantages of the liposome lipid bilayer, but addition of a surfactant that increases elasticity and deformation [81][33].3.1.4. Solid Lipid Nanoparticles (SLNs)
SLNs are nanoparticles containing a matrix composed of a solid lipid shell [83][35]. There are two methods to produce SLNs on a high scale: hot homogenization, and cold homogenization. In hot homogenization, CBD is dissolved in melted lipid and oil-in-water emulsions will be obtained after high-pressure homogenization. SLNs will form after cooling and recrystallization of the lipids in the emulsion. In cold homogenization, CBD is dissolved in melted lipid and after cooling and solidification, they are grounded to obtain lipid microparticles. Following this, the lipid microparticles are dispersed in a cold surfactant solution and then homogenized at room temperature to obtain SLNs [84][36]. SLNs were applied for the incorporation of curcumin and dexanabinol (a synthetic CBD-derivative) (CUR/CDB-SLNs). The mechanism associated with its antidepressant effects in corticosterone-induced cells and depression-mediated murine models was determined. In vivo results showed that CUR/CDB-SLNs significantly increased the dopamine/5-hydroxytryptamine release and decreased the corticosterone-triggered apoptosis in PC12 cells. In addition, in vivo results showed that CUR/CDB-SLNs increased levels of CB1 and CB1-mRNA, p-MEK1, and p-ERK1/2 protein expressions in the striatal and hippocampus region, thus exhibiting a neuroprotective effect, and can be used as a potential strategy for treating major depression [85][37]. Similar studies were performed by another group where they reported the antidepressant activity of CUR-DB SLNs in the wild-type (CBR1+/+) and CB1-knockout (CBR1–/–) mouse models of major depressive disorder [86][38]. CUR-DB SLNs enhanced the levels of mRNA and protein expressions, dopamine and norepinephrine release, and downregulated the expression of Rasgef1c and Egr1 genes, improving the locomotory functions of treated animals [86][38]. Similarly, other researchers enhanced the trans-membrane permeation of Δ8- THC by developing SLNs. The formulations exhibited sustained release behavior with increased drug loading studied in rabbit models. The size of SLNs assisted in the effective penetration of the encapsulated compound to the deeper ocular tissues even at a lower dose [86][38]. Nonetheless, SLNs allow low encapsulation loads, and in some cases, recrystallization risk exists. On the other hand, this system provides high efficiency, flexibility, and targetability [78][30].3.1.5. Nanostructured Lipid Carriers (NLCs)
NLCs can be produced by mixing liquid lipids with solid lipids based on the preparation method mentioned for SLNs. The obtained NLCs usually have smaller particle size compared to SLNs [87][39]. NLCs using stearic acid as a solid lipid and oleic acid as a liquid lipid, cetylpyridinium chloride, and Span 20 were developed for the nasal administration of CBD. Moreover, Pluronic F127 and Pluronic F68 as gelling polymers were added to the CBD-NLC dispersion to obtain a CBD-NLC-gel [88][40]. Monodisperse lipid nanocapsules (LNCs) as decomposable and biocompatible carriers were developed for CBD. LNCs prepared using the energetically-efficient phase inversion temperature (PIT) technique by caprylic-capric acid triglycerides, C18E15 polyethylene glycol (15) 12-hydroxystearate, soybean lecithin with 70% of phosphatidylcholine, and NaCl and after that CBD. were encapsulated into their oily core with high loading capacity [9][25]. Apart from decomposability and biocompatibility, NLCs offer high encapsulation loads, stability, and faster release than SLNs [78][30].3.1.6. Nanoemulsions
Nanoemulsions as stable colloidal delivery systems with nanosized particle structures, have a high ability in encapsulation, protection, and delivery of bioactive compounds [89][41]. Nanoemulsions have advantages such as the possibility of large-scale production and delivery of poorly-hydrophilic ingredients. However, this release is somehow rapid and poorly stable in gastric conditions [78][30]. CBD was incorporated into the oil phase of oil-in-water nanoemulsions using sonication and high-pressure homogenization. The obtained particle sizes were <200 nm and showed high stability after 30 days of production. Taskar et al. [90][42] reported the improved bioavailability and permeability of CBD through modified CBD-loaded nanoemulsions formed with the conjugation of amino acid and dicarboxylic acids analogs, CBD–divalinate–dihemisuccinate, and CBD–divalinate, respectively. It was noticed that the combination of both analogs significantly improved the ocular delivery of CBD, studied in a rabbit model in vivo. In addition, the CBD-dicarboxylic acids analogs exhibited better permeation of CBD through the ocular barriers. The fabrication parameters including particle size, surface charge, type of emulsifying agent, pH, and others play a crucial role in the effective delivery of CBD through nanoemulsions [90][42]. Chaudhari et al. [91][43] developed stable CBD-loaded nanoemulsions (120 nm) comprised of CBD distillates, soybean oil, and QNaturale (quillaja saponin containing commercial emulsifier) using high-pressure homogenization. These formulations could be efficiently used for diverse therapeutic applications [91][43]. In another study, castor oil-based nanoemulsions were developed for the effective delivery of hydrophobic drugs (fenofibrate or CBD) with improved bioavailability and stability. The method of preparation through a co-axial lamination mixer played a crucial role in regulating the stability of the nanoemulsions. Additionally, the concentration of oil and surfactant (Polysorbate 80) exhibited significant effects on the size, zeta potential, and polydispersity index of nanoemulsions [92][44]. CBD-loaded nanoemulsions comprised of CBD oil, vitamin E acetate, Tween-20, and ethanol were developed for improving the solubility and oral bioavailability of CBD. Results of the pharmacokinetic studies performed using a rat model showed improved oral bioavailability of CBD when administered as CBD-loaded nanoemulsions (50 mg/kg) [93][45]. Moreover, food-grade CBD-loaded nanoemulsions were prepared using canola oil or hemp seed oil, and medium-chain triacylglycerides. The exposure to light and the solution’s acidity were found to be two important factors responsible for maintaining the chemical stability of the CBD. As compared to bulk oil, nanoemulsions significantly improved the bioavailability of CBD [94][46]. Nanoemulsions as stable colloidal delivery systems can improve the loading, release properties, and therapeutic effects of CBD. The developed CBD-loaded nanocarriers showed improved solubility with high stability. The emulsions of medium-chain triglycerides, rapeseed oil, soybean oil, and trimyristin were prepared by high-pressure homogenization and showed high loading capacity. It was observed that the obtained emulsions showed a higher loading capacity than the suspension of SLNs based on trimyristin [95][47]. It was also observed that the absorption of CBD improved significantly compared with the medium-chain triglyceride (MCT) oil vehicle of CBD [96][48]. Several researchers investigated the effect of lipid type (medium- or long-chain triglycerides) combined with SNEDDS on the bioavailability of encapsulated CBD. It was concluded that although the type of lipid affects the solubility, bioavailability, and physicochemical properties of CBD, after incorporation in nanoemulsion formulation, its effect on the bioavailability of CBD is not easily predicted and needs more in vivo investigations [96][48].3.2. Polymeric/Biopolymeric Nanocarriers for CBD
Different polymeric or biopolymeric carriers have been used for the encapsulation of CBD. Moreover, several studies have developed a complex of natural and synthetic polyester-based nanomaterials as a potential carrier for effective delivery of therapeutics with improved characteristics [97][49]. Polyvinylpyrrolidone (PVP), Eudragit S-100 (ES), and Eudragit E-100 (EE) polymers were also used to fabricate nano-microfibers using electrospinning to encapsulate hemp extract for biomedical applications. Duran-Lobato et al. [98][50] developed a novel Δ-THC-loaded PLGA NPs (TPNPs) and evaluated its anticancer potential. TPNPs demonstrated a particle size of 300 nm with high entrapment efficiency (EE) and negative zeta potential. TPNPs improved the drug release properties and exhibited sustained release behavior. Various biological assays were performed to understand the mechanism of drug targeting and the results showed that TPNPs assisted in the targeted delivery of Δ-THC into the treated cells, showing its potential in cancer therapy [99][51]. Nanomicelles of Poloxamer 407 (P407) were used for encapsulation of CBD to enhance its bioavailability and biosafety. P407 is a triblock copolymer composed of (poly)ethylene oxide (PEO) and (poly)propylene oxide (PPO) sections. It has a PEO-PPO-PEO structure and can self-assemble into a micelle structure comprising a hydrophilic shell and hydrophobic core [99][51]. In another study, a cannabinoid derivative was encapsulated into the nanomicelles of poly(styrene-co-maleic anhydride) with an average size of 152 nm. The obtained NPs had the potential for high stability and prolonged blood circulation [52]. Hybrid nanocarriers are composed of two or more polymers in their structure. In this regard, the polymers usually stabilize each other and can better protect the encapsulated bioactives [100][53]. Wang et al. [100][53] fabricated CBD-entrapped polymeric hybrid NPs using two polymers (zein and whey protein) by the anti-solvent precipitation technique. Zein and whey protein-based polymeric NPs significantly improved the EE of CBD with superior stability. Results of FTIR studies confirmed hydrogen bond and hydrophobic forces in complexing zein and whey protein. In addition, the zein-whey protein-CBD NPs exhibited superior antioxidant activity and improved in vitro release of CBD. The results indicated that hybrid NPs of zein and whey protein could better protect CBD against thermal degradation and UV in comparison to zein NPs [100][53]. The Millard conjugate of whey protein-maltodextrin (WP-MD) was prepared using controlled dry heating and complexed with rosmarinic acid (RA) to be used as a stabilizing agent for oil-in-water emulsions for incorporating CBD. The emulsion that was stabilized with WP-MD-RA indicated the most protection efficiency of CBD against thermal degradation and UV in comparison to the emulsion containing WP-MD, WP, and WP-RA. Sosnik et al. [101][54] fabricated hybrid polymeric micelles (PMs) comprised of CBD, chitosan (CS), and poly-(vinyl alcohol) (PVA). The hybrid PMs were found to be biocompatible, stable, and exhibited high penetrability of CBD within the human corneal epithelial cells [102][55]. Low-density lipoprotein (LDL) of yolk was complexed with carboxymethyl cellulose (CMC) as a carrier for nanoencapsulation of CBD using emulsification at neutral pH. Defatting of LDL had a significant effect on its dispersibility. It was observed that a carrier oil with low viscosity is favored and triglyceride with medium chain gives rise to better nanoencapsulation in comparison to vegetable oils such as high oleic soybean oil. A novel topical formulation in the form of cryogel comprising 2-hydroxyethyl cellulose and β-cyclodextrin was developed for the effective delivery of CBD. The cryogels exhibited a bi-phasic release pattern, with an initial burst release (first 3 h) followed by slower release. The cryogels showed dose-dependent cytotoxicity against human cancer cells (MJ and HUT-78), which showed its potential in the management of skin neoplastic diseases [102][55]. In another study, Momekova et al. [103][56] developed a water-soluble nanocomposite cryogel containing 2-hydroxyethyl cellulose and CBD-incorporated polymeric micelles. Indeed, CBD was first incorporated into the polymeric core-shell micelles, and then the obtained polymeric micelles were encapsulated into 2-hydroxyethyl cellulose cryogel carriers through UV-assisted cryotropic gelation [103][56]. Pickering emulsions (PEs), as surfactant-free emulsions, are a group of eco-friendly carriers for bioactive compounds. Chitosan/gum rabic (CS/GA) particles were used for the stabilization of PEs, and the degree of deacetylation (DDA) of CS was evaluated on the stability of PEs. CBD was dissolved in the oil phase of the emulsion and then added to CS/GA dispersion and homogenized. The results indicated that by increasing the DDA, the stability of PEs was increased. The skin absorption of incorporated CBD was higher than its permeation, which shows its potential use for dermal delivery of CBD [104][57]. In another study, a fabricated scaffold of a gelatin/nano-hydroxyapatite (G/nHAp) delivered CBD-loaded PLGA microspheres to specific sites of bone defects in a rat model [105][58]. Moreover, the lipid- or polymer-based nanocarrier approaches significantly improve the release behavior of CBD at the targeted site with improved loading, leading to increased bioavailability and decrease in toxicity (Figure 3).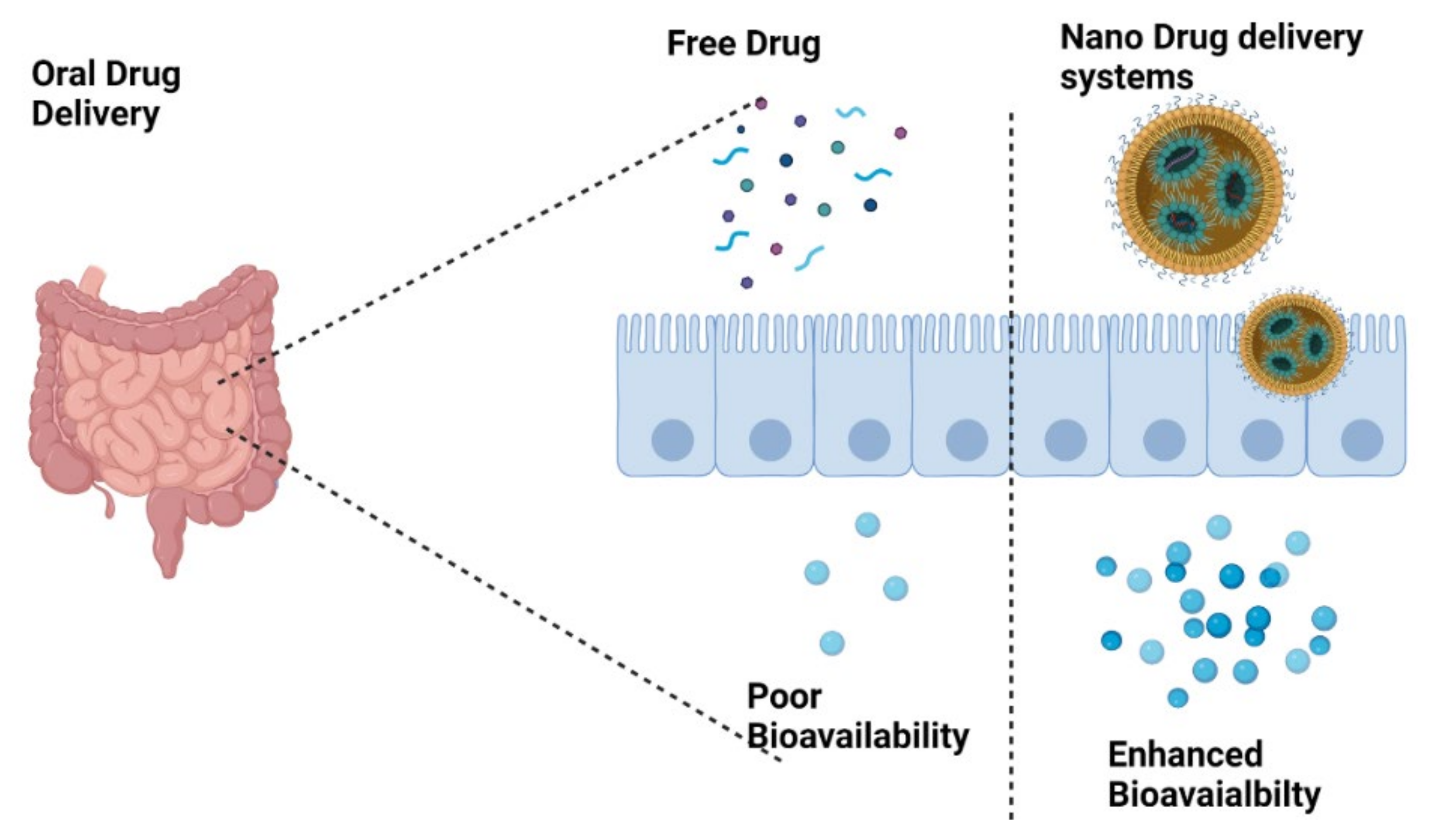
Figure 3. Schematic representation of release mechanisms from nanocarriers permeating via various biological barriers.
References
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture 2021, 11, 384.
- Touw, M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34.
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430.
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21.
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.-N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155.
- Basas-Jaumandreu, J.; de Las Heras, F.X.C. GC-MS Metabolite Profile and Identification of Unusual Homologous Cannabinoids in High Potency Cannabis sativa. Planta Med. 2020, 86, 338–347.
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331.
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804.
- Martin, J.H.; Schneider, J.; Lucas, C.J.; Galettis, P. Exogenous Cannabinoid Efficacy: Merely a Pharmacokinetic Interaction? Clin. Pharmacokinet. 2017, 57, 539–545.
- Harshita; Barkat, M.A.; Das, S.S.; Pottoo, F.H.; Beg, S.; Rahman, Z. Lipid- Based Nanosystem As Intelligent Carriers for Versatile Drug Delivery Applications. Curr. Pharm. Des. 2020, 26, 1167–1180.
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219.
- Aladić, K. Cold Pressing and Supercritical CO2 Extraction of Hemp (Cannabis sativa) Seed Oil. Chem. Biochem. Eng. Q. 2015, 28, 481–490.
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176.
- Di Bello, M.P.; Bloise, E.; Mazzetto, S.E.; Mele, G. Formulation and Chemical Stability in Aqueous Media of Cannabidiol Embedded in Cardanol-Based Nanovesicles. ACS Sustain. Chem. Eng. 2017, 5, 8870–8875.
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31.
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150.
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016, 112, 119–127.
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163.
- Sherman, J.J.; Riche, D.M.; Warren, C.P. Cannabidiol Oral Solution: Challenges as a Treatment for Seizure Syndromes. The J Nurse Pract. 2020, 16, 210–212.
- Leo, A.; Russo, E.; Elia, M. Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol. Res. 2016, 107, 85–92.
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The Endocannabinoid System and Cannabidiol: Past, Present, and Prospective for Cardiovascular Diseases. Pharmaceuticals 2021, 14, 936.
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322.
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740.
- Jha, N.K.; Arfin, S.; Jha, S.K.; Kar, R.; Dey, A.; Gundamaraju, R.; Ashraf, G.M.; Gupta, P.K.; Dhanasekaran, S.; Abomughaid, M.M.; et al. Re-establishing the comprehension of phytomedicine and nanomedicine in inflammation mediated cancer signaling. Semin Cancer Biol. 2022, 86, 1086–1104.
- Aparicio-Blanco, J.; Sebastián, V.; Benoit, J.P.; Torres-Suárez, A.I. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. Eur. J. Pharm. Biopharm. 2018, 134, 126–137.
- Atsmon, J.; Cherniakov, I.; Izgelov, D.; Hoffman, A.; Domb, A.J.; Deutsch, L.; Deutsch, F.; Heffetz, D.; Sacks, H. PTL401, a New Formulation Based on Pro-Nano Dispersion Technology, Improves Oral Cannabinoids Bioavailability in Healthy Volunteers. J. Pharm. Sci. 2018, 107, 1423–1429.
- Das, S.S.; Hussain, A.; Verma, P.R.P.; Imam, S.S.; Altamimi, M.A.; Alshehri, S.; Singh, S.K. Recent Advances in Liposomal Drug Delivery System of Quercetin for Cancer Targeting: A Mechanistic Approach. Curr Drug Deliv. 2020, 17, 845–860.
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control. Release 2017, 266, 1–7.
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. Methods Mol. Boil. 2010, 605, 29–50.
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain Pi 2020, 161, 2191–2202.
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27.
- Shilo-Benjamini, Y.; Cern, A.; Zilbersheid, D.; Hod, A.; Lavy, E.; Barasch, D.; Barenholz, Y. A Case Report of Subcutaneously Injected Liposomal Cannabidiol Formulation Used as a Compassion Therapy for Pain Management in a Dog. Front. Vet. Sci. 2022, 9, 550.
- Benson, H.A. Transfersomes for transdermal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737.
- Moqejwa, T.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E. Development of Stable Nano-Sized Transfersomes as a Rectal Colloid for Enhanced Delivery of Cannabidiol. Pharmaceutics 2022, 14, 703.
- Balaga, V.K.R.; Pradhan, A.; Singh, S.; Dev, A. An overview on nanoparticulate drug delivery system for its specific and targeted effects in various diseases. In Nanoparticle and Nanocarrier Based Pharmaceutical Formulations; Bentham Science Publishers Pte. Ltd.: Singapore, 2022; pp. 55–92.
- Müller, R.; Radtke, M.; Wissing, S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128.
- He, X.; Yang, L.; Wang, M.; Zhuang, X.; Huang, R.; Zhu, R.; Wang, S. Targeting the Endocannabinoid/CB1 Receptor System for Treating Major Depression Through Antidepressant Activities of Curcumin and Dexanabinol-Loaded Solid Lipid Nanoparticles. Cell. Physiol. Biochem. 2017, 42, 2281–2294.
- Punyamurthula, N.S.; Adelli, G.R.; Gul, W.; Repka, M.A.; ElSohly, M.A.; Majumdar, S. Ocular Disposition of ∆8-Tetrahydrocannabinol from Various Topical Ophthalmic Formulations. AAPS PharmSciTech 2016, 18, 1936–1945.
- Fang, J.-Y.; Fang, C.-L.; Liu, C.-H.; Su, Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanopar-ticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640.
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain. Eur. J. Pharm. Sci. 2021, 159, 105698.
- Das, S.S.; Sarkar, A.; Chabattula, S.C.; Verma, P.R.P.; Nazir, A.; Gupta, P.K.; Ruokolainen, J.; Kesari, K.K.; Singh, S.K. Food-Grade Quercetin-Loaded Nanoemulsion Ameliorates Effects Associated with Parkinson’s Disease and Cancer: Studies Employing a Transgenic C. elegans Model and Human Cancer Cell Lines. Antioxidants 2022, 11, 1378.
- Taskar, P.S.; Patil, A.; Lakhani, P.; Ashour, E.; Gul, W.; ElSohly, M.A.; Murphy, B.; Majumdar, S. Δ9-Tetrahydrocannabinol Derivative-Loaded Nanoformulation Lowers Intraocular Pressure in Normotensive Rabbits. Transl. Vis. Sci. Technol. 2019, 8, 15.
- Chaudhari, V.S.; Gawali, B.; Saha, P.; Naidu, V.; Murty, U.S.; Banerjee, S. Quercetin and piperine enriched nanostructured lipid carriers (NLCs) to improve apoptosis in oral squamous cellular carcinoma (FaDu cells) with improved biodistribution profile. Eur. J. Pharmacol. 2021, 909, 174400.
- Erfle, P.; Riewe, J.; Bunjes, H.; Dietzel, A. Goodbye fouling: A unique coaxial lamination mixer (CLM) enabled by two-photon polymerization for the stable production of monodisperse drug carrier nanoparticles. Lab Chip 2021, 21, 2178–2193.
- Nakano, Y.; Tajima, M.; Sugiyama, E.; Sato, V.H.; Sato, H. Development of a Novel Nano-emulsion Formulation to Improve Intestinal Absorption of Cannabidiol. Med. Cannabis Cannabinoids 2019, 2, 35–42.
- Zheng, H.; Chen, B.; Rao, J. Nutraceutical potential of industrial hemp (Cannabis sativa L.) extracts: Physicochemical stability and bioaccessibility of cannabidiol (CBD) nanoemulsions. Food Funct. 2022, 13, 4502–4512.
- Francke, N.; Schneider, F.; Baumann, K.; Bunjes, H. Formulation of Cannabidiol in Colloidal Lipid Carriers. Molecules 2021, 26, 1469.
- Das, S.S.; Singh, S.K.; Verma, P.R.P.; Gahtori, R.; Sibuh, B.Z.; Kesari, K.K.; Jha, N.K.; Dhanasekaran, S.; Thakur, V.K.; Wong, L.S.; et al. Polyester nanomedicines targeting inflammatory signaling pathways for cancer therapy. Biomed Pharmacother. 2022, 154, 113654.
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and In Ovo Assessment. Pharmaceutics 2020, 12, 439.
- Durán-Lobato, M.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Martín-Banderas, L. Receptor-targeted nanoparticles modulate cannabinoid anticancer activity through delayed cell internalization. Sci. Rep. 2022, 12, 1297.
- Rao, Y.; Li, R.; Liu, S.; Meng, L.; Wu, Q.; Yuan, Q.; Liang, H.; Qin, M. Enhanced bioavailability and biosafety of cannabidiol nanomicelles for effective anti-inflammatory therapy. Particuology 2021, 69, 1–9.
- Greish, K.; Mathur, A.; Al Zahrani, R.; Elkaissi, S.; Al Jishi, M.; Nazzal, O.; Taha, S.; Pittalà, V.; Taurin, S. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J. Control. Release 2018, 291, 184–195.
- Wang, C.; Cui, B.; Sun, Y.; Wang, C.; Guo, M. Preparation, stability, antioxidative property and in vitro release of cannabidiol (CBD) in zein-whey protein composite nanoparticles. LWT 2022, 162, 113466.
- Sosnik, A.; Ben Shabo, R.; Halamish, H.M. Cannabidiol-Loaded Mixed Polymeric Micelles of Chitosan/Poly(Vinyl Alcohol) and Poly(Methyl Methacrylate) for Trans-Corneal Delivery. Pharmaceutics 2021, 13, 2142.
- Momekova, D.; Danov, Y.; Momekov, G.; Ivanov, E.; Petrov, P. Polysaccharide Cryogels Containing β-Cyclodextrin for the Delivery of Cannabidiol. Pharmaceutics 2021, 13, 1774.
- Momekova, D.; Ivanov, E.; Konstantinov, S.; Ublekov, F.; Petrov, P.D. Nanocomposite Cryogel Carriers from 2-Hydroxyethyl Cellulose Network and Cannabidiol-Loaded Polymeric Micelles for Sustained Topical Delivery. Polymers 2020, 12, 1172.
- Sharkawy, A.; Barreiro, F.; Rodrigues, A. Pickering emulsions stabilized with chitosan/gum Arabic particles: Effect of chitosan degree of deacetylation on the physicochemical properties and cannabidiol (CBD) topical delivery. J. Mol. Liq. 2022, 355, 118993.
- Kamali, A.; Oryan, A.; Hosseini, S.; Ghanian, M.H.; Alizadeh, M.; Eslaminejad, M.B.; Baharvand, H. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater. Sci. Eng. C 2019, 101, 64–75.
More
