Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Bahar Faramarzi.
Sphingolipids have attracted significant attention due to their pivotal role in cellular functions and physiological diseases. A valuable tool for investigating the characteristics of sphingolipids can be represented via Fourier-transform infrared (FT-IR) spectroscopy, generally recognized as a very powerful technique that provides detailed biochemical information on the examined sample with the unique properties of sensitivity and accuracy.
- sphingolipids
- sphingomyelin
- sphingosine
- ceramide
- human cells
- FT-IR spectroscopy
1. Introduction
Lipids are a heterogeneous organic group of biomolecules that are insoluble in water due to the hydrocarbon nature of a large part of their structure. Lipids have several essential biological functions: they represent structural components of membranes, they serve as storage and transport for energy-rich molecules, they act as a protective coating on the surface of many organisms, and they also participate in the recognition of specific characters. Some of them have significant biological activity, usually typified by vitamins and hormones. Although lipids are a distinct class of biomolecules, they are often bound with components of other biomolecules to form hybrid molecules, such as glycolipids containing carbohydrates, lipids, and lipoproteins, which contain lipids and proteins. In these biomolecules, their components’ characteristic chemical and physical properties mix to perform specialized biological functions [1,2,3][1][2][3].
Recently, sphingolipids (SLs) received significant attention due to their multiple roles in cellular functions, such as cell migration and adhesion, formation of membrane domain, DNA damage response, senescence, aging autophagy, and apoptosis. SLs also have fundamental functions in cancer cell biology, diabetes, cardiovascular diseases, neurodevelopment, neurodegeneration, and in biological processes related to tissue and bones [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21]. Given the relevance of this class of lipids, many different experimental techniques have been exploited for their characterization. Thin-layer chromatography (TLC), high-pressure liquid chromatography (HPLC), gas chromatography (GC), and capillary electrophoresis (CE) are the most primarily used techniques used to characterize the structural diversity of SLs [5,22,23][5][22][23]. These techniques have allowed for the development of a sphingolipidomics approach in which quantitative structural analysis of all SLs, or at least all members of a substantial subgroup, can be pursued in large samples [5,24,25,26][5][24][25][26].
Several studies about SLs used methodologies based on NMR and vibrational techniques, such as Raman and Fourier-transform infrared (FT-IR) spectroscopies [22]. This last technique represents a unique tool thanks to its sensitivity in acquiring information on biological samples [27,28,29,30,31,32,33][27][28][29][30][31][32][33] and the effects of their interactions with external agents [34,35,36,37][34][35][36][37]. In addition, FT-IR spectroscopy has been also adopted for differentiating between benign and malignant tumors in the colon [38[38][39],39], breast tissues [40[40][41],41], and prostate [42,43][42][43]. The analysis of biofluids (saliva, serum, urine, and blood) represent another interesting field of application of this technique [44[44][45],45], even though particular attention should be paid to the contribution of water content that can interfere with the signal coming from the examined samples [46,47][46][47].
As far as concerns lipids, great attention has been devoted to the acquisition of infrared spectra of lipids. as is evident from [22,48,49,50,51,52][22][48][49][50][51][52] and the references therein. The huge amount of work performed in this field has made available a certain number of databases dedicated to the FT-IR spectroscopy of lipids and similar compounds. In Table 1, the most cited ones are reported.
Table 1.
Online databases with information on lipids and lipidomics.
| Source | Url | Notes |
|---|---|---|
| Lipid Bank—Japanese Conference on the Biochemistry of Lipids (JCBL) | https://lipidbank.jp (accessed on 1 February 2023) | LipidBank is a free database of natural lipids including fatty acids, glycerolipids, SLs, steroids, and various vitamins.The database contains more than 6000 unique molecular structures, their lipid names, and spectral and literature information. |
| NIST Chemistry WebBook | https://webbook.nist.gov (accessed on 1 February 2023) | The NIST Chemistry WebBook provides access to: thermochemical data; IR spectra, mass spectra, UV/Vis spectra, and gas chromatography data. It is possible to search for data on specific compounds based on name, chemical formula, CAS registry number, molecular weight, chemical structure, or selected ion energetics and spectral properties. |
| Spectral Database for Organic Compounds (SDBS) | https://sdbs.db.aist.go.jp (accessed on 1 February 2023) | SDBS is an integrated spectral database system for organic compounds, which includes 6 different types of spectra: an electron impact mass spectrum (EI-MS), a Fourier-transform infrared spectrum (FT-IR), a 1H nuclear magnetic resonance (NMR) spectrum, a 13C NMR spectrum, a laser Raman spectrum, and an electron spin resonance (ESR) spectrum. |
Given the potentialities of FT-IR spectroscopy in lipid characterization and quantification, many researchers have adopted FT-IR for investigating particular aspects of SLs and their role in different biological frameworks.
2. Basic Principles of FT-IR Spectroscopy
FT-IR spectroscopy analyzes the spectral infrared radiation absorbed by a sample. The energy of the absorbed infrared radiation equates the energy needed to transition between the vibrational states of functional groups. The radiation intensity absorbed by a sample produces the infrared absorption spectrum, in which there are peaks or bands related to a particular mode of the vibrations that is characteristic of functional groups in the sample [27,37][27][37]. The main component of a modern FT-IR spectroscopic apparatus is a Michelson interferometer (see Figure 1 and [37] for further details), which substitutes the monochromator that was present in the old infrared spectrometers. FT-IR spectrometers enable the collection of absorption spectra quickly and precisely by obtaining qualitative and quantitative information on the structure of the compounds analyzed using modern software algorithms.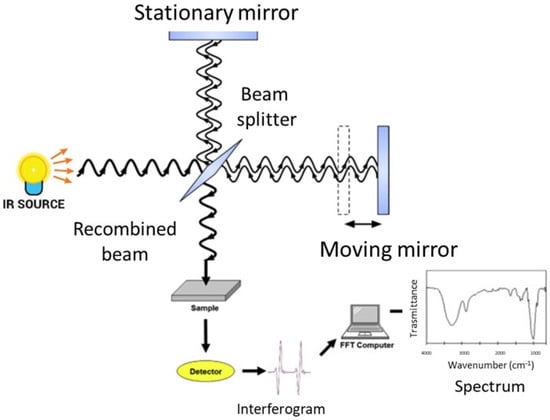
Figure 1.
Schematic representation of an FT-IR spectrometer.
3. Experimental Aspects of FT-IR Spectroscopy
There are three main infrared spectroscopy sampling modes: transmission, reflectance, and attenuated total reflection (ATR), each with advantages and disadvantages [31,72][31][56]. For lipid analysis, the most largely used approaches are transmission and ATR modes. For transmission measurements, the samples are placed between two IR transparent windows. Generally, CaF2, BaF2, and KBr windows are used together with a proper sample holder in which the Teflon spacer can obtain the appropriate thickness, typically around 6–10 µm [73][57]. As an alternative, the samples can be included within a KBr matrix and pressed to form a pellet that can be subsequently analyzed after being dried [74][58]. The ATR approach is based on the presence of an evanescent wave at the reflecting interface between the sample and the crystal [75][59]. When a radiation beam penetrates inside a crystal, and the angle of incidence at the interface between the sample and crystal is greater than the critical angle, which is given by the refractive indices of the two surfaces, the beam penetrates a fraction of a wavelength beyond the reflecting surface, and when it comes into close contact with a material that selectively absorbs radiation, the beam loses energy at the wavelength where the material absorbs. The spectrometer measures and plots the attenuated radiation as a function of the wavelength, yielding the sample’s absorption spectral characteristics. ATR employs crystal materials, such as diamond, ZnSe, and ZnS, in contact with the sample under investigation. A pressure plate is needed to ensure the connection between the samples and the crystal. Except for diamond objectives, excessive pressure can harm some crystals [76,77][60][61].4. FT-IR Characterization of SLs
Before discussing the principal results reported in the literature about FT-IR studies on SLs, it is worthwhile to mention the fundamental paper of Fringeli and Gunthard [79][62] that provides the basis for all subsequent studies about lipids components in biological samples. Some other authors give relevant contributions in this field [75,80,92,93,94][59][63][64][65][66]. In Table 2, an almost exhaustive list of the peaks present in lipid samples is reported together with their assignments.Table 2.
| Sphingolipid Compound | Structure |
|---|---|
| Ceramide (Cer) | 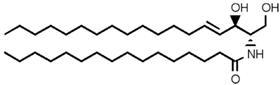 |
| Sphingosine 1-phosphate (S1P) | 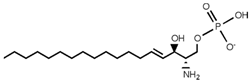 |
| Sphingosine (SP) | 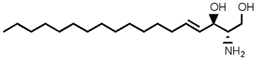 |
| Ceramide-1-phosphate (C1P) | 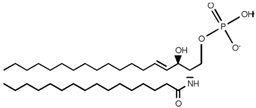 |
| Dihydroceramide | 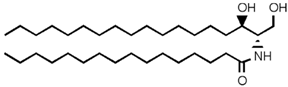 |
| Sphingomyelin (SM) |
| Peaks Position (cm−1) |
Assignments |
|---|---|
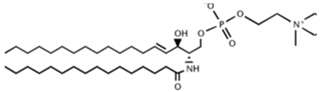 | |
| Galactosylceramide | |
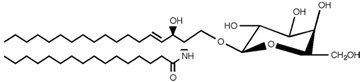 |
|
| Lactosylceramide | 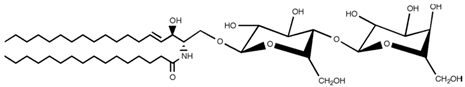 |
| Glucosylceramide | 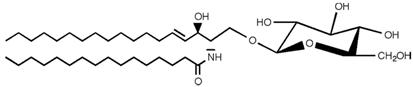 |
| 892 | C=C bending (fatty acid) |
| 2916–2925 | |
| CH | |
| 2 | |
| asymmetric stretching (mainly lipids, | |
| with little contribution from proteins, carbohydrates, and nucleic acids) | |
| 2956–2970 | |
| CH | |
| 3 | asymmetric stretching (lipids, fatty acids, protein side chains, with some contribution from carbohydrates and nucleic acids) |
| 3007–3015 | C-H stretching (lipids, unsaturated fatty acids) |
- (a)
-
Sphingomyelin (SM)The paper of Villalain et al., cited in Table 4, is particularly interesting due to the joint use of differential scanning calorimetry and FT-IR spectroscopy to investigate the molecular interactions between PC and SM. The authors observed that no significant change in the wavenumber position for the antisymmetric and symmetric CH2 stretching is present when SM is added to PC at temperatures above or below the phase transition and then they demonstrated that the gauche ratio to all-trans is not affected by SM and PC interaction. The authors also examined the characteristics of the C=O stretching band of PC located at 1735 cm−1, evidencing that the phosphate group of SM takes part in hydrogen bonding between the molecules of SM and possibly PC. Particular attention was also given to the Amide I contribution at 1635 cm−1 and to bands related to the phosphate group and located at 1220 and 1080 cm−1 [99][71]. Dreissig et al. investigated the different SLs and phospholipids that are present in lipids extracted from the porcine brain. They presented the FT-IR spectra of some SLs, phospholipids, and neutral lipids. If the SM spectrum is considered in detail, it is possible to recognize the contribution of different vibration modes reported in Table 3 and in Table 2 of [103][75]. In the high wavenumber region related to hydrocarbon chains, peaks related to CH2 asymmetric and symmetric stretching are positioned at 2924 and 2852 cm−1, respectively. In the spectral region related to the lipid polar headgroup, contributions attributed to C=O stretching (1647 cm−1), N-H bending (1545 cm−1), CH2 bending (1466 cm−1), CH3 symmetric bending (1378 cm−1), PO-2 asymmetric stretching (1240 cm−1), PO-2 symmetric stretching (1090 cm−1), and C-O-C stretching (1055 cm−1) can be noticed. In [103][75], the authors examined the difference in band position and intensities in the spectra of the different commercial samples. They also prepared lipid mixtures to train and validate a quantification model for determining the composition of brain lipid extracts. The results of the PLS regression of FT-IR spectra were successfully related to the lipid quantification obtained by using TLC. Even though the FT-IR spectroscopic approach presented in this paper cannot be generally used for the quantitative analysis of all lipids, it might contribute to the diagnosis of brain tumors by evaluating the changes occurring in the lipid composition of tumor cells [103][75]. The papers of Gasper et al. [104,105][76][77] represent a typical example of the use of FT-IR spectroscopy for studying the effects of drugs on SLs, a topic largely investigated by using this vibrational technique. In the present case, the authors aim to examine the number of changes in lipids in PC-3 cells exposed to sub-lethal levels of ouabain, a well-known cardiotonic steroid that demonstrates an anti-cancer activity in vitro and in vivo that indicates the possibility of adopting these compounds as chemotherapeutic agents in oncology. The authors examined the different spectra obtained between the average spectra for lipid extracts related to different experimental conditions. In [104][76], three curves are reported: the first is related to the changes occurring in the first 6 h after the treatment, the second is related to the changes between 24 and 6 h of treatment and the last one concerns the changes between 36 and 24 h of treatment. The most relevant changes for lipids are present between 6 and 24 h. Principal component analysis (PCA) was also performed for evaluating the differences among the FT-IR spectra related to different experimental conditions. According to the findings of [104[76][77],105], FT-IR spectroscopy not only detects cellular changes caused by ouabain but also qualitatively assesses the evolution of these alterations during therapy. Ouabain’s overall biological effect on PC-3 cell lipids increased SM and decreased PC [104,105][76][77]. A particularly interesting aspect of FT-IR spectroscopy is related to the possibility of its use for the quantitative determination of a single component in a mixture. This possibility has been evidenced by the paper of Derenne et al. [96][68], which developed PLS algorithms for quantifying lipids in complex mixtures of lipids. The lipid composition obtained via FT-IR spectroscopy was first validated by using HPLC and used for determining the lipid composition of lipids extracted from cells exposed to four different drugs. These treatments did not cause significant changes in the polar headgroups’ spectral region. However, the authors suggested that their PLS models appear to be reliable and can be helpful for routine analysis [96][68]. (b) Ceramide (Cer) Moore et al. [108][80] investigated the conformational order and phase behavior of hydrated Cers to provide the first complete investigation of the intermolecular and intramolecular chain and headgroup interactions in hydrated non-hydroxy fatty acid (NFA) and hydroxy fatty acid (HFA) Cers. In particular, the authors examined the temperature dependence of the methylene stretching, scissoring, and rocking mode frequencies. This study indicates the occurrence of two relevant phase transitions, one around 60 °C and the other around 80 °C. The behavior of the Amide I and II modes shows different contributions from NFA and HFA Cers. The results reported in this paper were also of relevance to the domain mosaic model of the stratum corneum lipid barrier that was proposed in the seventies of the last century. Another paper addressing the analysis of the conformational order of Cer by using FT-IR spectroscopy is reported in [114][81]. In addition, these authors investigated the dependence on temperature of the conformational stability of Cers. They studied different Cers and mixtures and polar–nonpolar lipid interactions. Their study evidenced the impact of the polar headgroup on the conformation of the hydrocarbon chains when the temperature increases, with the role of the endogenous molecules also being more prominent. More recently, de Arada et al. [118][82] used FT-IR spectroscopy to examine the interactions of polar headgroups from Cers and SM. These authors studied four regions of the FT-IR spectra of these compounds: C-H stretching and CH2 scissoring vibrations, the Amide I region, and the phosphate vibration range. The study of the two latter areas was the novelty of the de Arata et al. paper. The temperature dependence of hydrated samples of pure SM and SM–Cer mixtures was examined and compared with the results of differential scanning calorimetry measurements. The data from the hydrocarbon chain region show a transition in agreement with previous observations. The results related to the Amide I region are more interesting since they evidence that SM and Cer carbonyl groups strongly interact, probably through H bonds. Furthermore, the results pertaining to the phosphate group suggest a relevant role of H bonds in the interaction between SM and Cer. The results of [118][82] evidence that SM and Cer can have an interaction through their polar headgroups that is differently from what occurs to other lipids. (c) Sphingosine (SP) and sphingosine 1-phosphate (S1P) The contribution reported in [119][83] is related to the joint use of differential scanning thermometry and FT-IR spectroscopy for investigating the effect of SP and stearylamine on the interaction of SP with calcium. Additionally, in this case, spectroscopic observations confirm the phase transition observed by using differential scanning thermometry. The inspection of the ester C=O stretching mode appearing as a broad band at 1734 cm−1 suggests that the amino bases do not introduce hydrogen bonding with the C=O group and do not modify its hydration state. The same evidence is obtained by considering the band related to the phosphate group located at 1220 cm−1. The degree of dehydration of the phosphate group can also be quantitatively determined by using a partial least-squares multivariate statistical analysis. A further study from the same research group [120][84] is devoted to the use of FT-IR for quantitative analysis of the dehydration process of the phosphatidylserine phosphate group in the presence of Ca2+ caused by various molecules, such as diacylglycerol, SP, and stearylarnine, by adopting a partial least-squares statistical method. FT-IR can also be used for estimating the apparent pKa of lipid carboxyl groups. The absorbance signals given by the protonated and the unprotonated forms of the specific group under investigation can be determined. In so doing, it is possible to evidence that diacylglycerol increases the dehydration of the phosphate group due to Ca2+ while the amino-bases SP and stearylamine avoid the dehydration of the phosphate group. The paper of Derenne et al. [96][68] that has been cited in the previous table also gives interesting results for SP and S1P. (d) FT-IR Lipidomic studies involving sphingolipids It is worth noting that, recently, great attention has been devoted to two aspects of FT-IR spectroscopy technology to broaden its field of application further. The former of these aspects are related to the development of experimental approaches allowing the characterization of a considerable number of samples by developing a high-throughput approach [127,128,129][85][86][87]. The second concerns the “omics” aspect. Usually, this term is used for indicating the analysis of genes (genomics), proteins (proteomics), and metabolites (metabolomics), but also the analysis of all lipids and the molecules with which they interact, and their function within biological systems assume a particular relevance since lipidomics represent one of the most critical aspects of modern analytical biochemistry. The most general techniques used in lipidomics are the techniques of TLC, HPLC, GC, and CE mentioned in the Introduction of the present paper; however, also in this framework, FT-IR spectroscopy is increasingly appreciated [22,130,131][22][88][89]. Considering sphingolipids, Ramos-Garcia et al. [25] showed that FT-IR analysis could provide a qualitative and quantitative biochemical characterization of isolated exosomes, allowing for a fast and direct quantification of the total lipid content and evidencing sphingolipid contribution. Another contribution in this field has been given by Guleken et al. that individuated changes in sphingolipid metabolism in the blood serum of endometriosis-affected patients [24,26][24][26].Table 4.Summary of some relevant results obtained on SM by using different FT-IR spectroscopy approaches.
References Lipid Extraction
Method/Sample DetailsSpectra Collection Geometry Aim Main Findings [99][71] Commercial
samplesTransmission geometry using CaF2 windows To investigate the molecular interactions between SM and PC in phospholipid vesicles. The changes in the acyl chains and SM, conformation induced by PC are observed. 1050–1070 [100][72] C-O-C stretching (nucleic acids and phospholipids) Commercial
samplesTransmission geometry using CaF2 windows To study the effects of temperature and pressure on structural and conformational properties
of PC/SM/cholesterol model raft mixtures.The conformational properties of the lipid systems are monitored by examining the positions and
intensities of infrared absorption bands.1085–1090 [101,102][73][ PO-2 symmetric stretching (nucleic acids and phospholipid) 74] Rat brain tissue samples Transmission geometry using CaF2 windows To examine the spatial distribution of molecular changes associated with C6 glioma progression. 1224–1240 PO-2 asymmetric stretching (nucleic acids and phospholipid) The concentrations of SM, nucleic acids, PS, and glucocerebroside are significantly affected during C6 glioma development. Lipids extracted from brain tissues using
Folch and Bligh and Dyer methods.Transmission
KBr pellets1343 CH2 wagging bending (phospholipid, fatty acid, and triglyceride) [103][75] To analyze the lipid extracts from the brain to identify their composition. Lipid content can be evaluated via FT-IR spectroscopy, which may improve the differential diagnosis of brain cancers. [104,[76105]][77] Commercial samples and lipids extracted from PC 3 cells using Bligh and Dyer method. ATR To analyze the changes in the lipidome of prostate cancer PC-3 cells after exposure to sub-lethal ouabain levels. Lipid alterations induced by ouabain can be identified by variations in the ester/choline/phosphate ratios in FT-IR spectra. 1367 [96][68] CH3 symmetric bending (lipids) Commercial samples and lipids extracted from PC-3 cells using Bligh and Dyer method. Micro-ATR To develop PLS models based on FT-IR spectra to determine the changes in the amounts of different lipids in extracts from PC-3 cells treated with four antitumor drugs. After treatments with anticancer drugs, the spectral region of the polar headgroups of samples did not show any noticeable alterations. However, the developed PLS models can be used for high-throughput measurements. 1392–1400 [106][78] CH2 asymmetric bending, COO- stretching (proteins and fatty acids) Commercial
samplesATR To investigate the changes occurring in detergent-resistant membranes (DRM) extracted from human breast cancer cells when treated with the omega-3 fatty acid docosahexaenoic acid. FT-IR spectroscopy and multivariate analysis enable to monitor of the changes in the composition of DRMs. This approach can be useful for the label-free characterization of lipid components in cells. 1445–1470 [107][79] CH2 bending (mainly lipids and phospholipids,
with little contribution from proteins)Commercial
samplesTransmission geometry using CaF2 1456–1467 CH3 bending (lipids, cholesterol, and proteins) windows To examine the interaction profile of carboplatin at varying concentrations with SM multilamellar vesicles. 1545–1549 N-H bending (lipids) 1660–1670 C=C stretching (lipids, fatty acids) 1730–1750 C=O stretching (fatty acid ester, triglycerides, and cholesterol esters) 2850–2865 CH2 symmetric stretching (lipids, fatty acids) Carboplatin affects the phase transition, enthalpy, the cooperativity parameter, the phase transition temperature, the lipid order, the lipid fluidity, and the hydrogen state of specific groups in hydrophilic parts of the examined samples. 2870–2874 CH3 symmetric stretching (protein side chains, lipids,
with some contribution from carbohydrates and nucleic acids)
References
- Muro, E.; Atilla-Gokcumen, G.E.; Eggert, U.S. Lipids in cell biology: How can we understand them better? Mol. Biol. Cell 2014, 25, 1819–1823.
- van Meer, G. Cellular lipidomics. EMBO J. 2005, 24, 3159–3165.
- Zalba, S.; Ten Hagen, T.L. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57.
- Breslow, D.K.; Weissman, J.S. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell 2010, 40, 267–279.
- Haynes, C.A.; Allegood, J.C.; Park, H.; Sullards, M.C. Sphingolipidomics: Methods for the comprehensive analysis of sphingolipids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2696–2708.
- Gennis, R.B. Biomembranes: Molecular Structure and Function; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013.
- Chapman, D. (Ed.) Biomembrane Structure and Function; Springer: Berlin/Heidelberg, Germany, 1983.
- Cullis, P.R.; Hope, M.J. Chapter 1 Physical properties and functional roles of lipids in membranes. In New Comprehensive Biochemistry; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 20, pp. 1–41.
- Lizardo, D.Y.; Parisi, L.R.; Li, N.; Atilla-Gokcumen, G.E. Noncanonical roles of lipids in different cellular fates. Biochemistry 2018, 57, 22–29.
- Merrill, A.H., Jr.; Sandhoff, K. Sphingolipids: Metabolism and cell signaling. New Compr. Biochem. 2002, 36, 373–407.
- Holm, L.J.; Krogvold, L.; Hasselby, J.P.; Kaur, S.; Claessens, L.A.; Russell, M.A.; Mathews, C.E.; Hanssen, K.F.; Morgan, N.G.; Koeleman, B.P. Abnormal islet sphingolipid metabolism in type 1 diabetes. Diabetologia 2018, 61, 1650–1661.
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96.
- Duan, R.-D.; Nilsson, Å. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res. 2009, 48, 62–72.
- Iessi, E.; Marconi, M.; Manganelli, V.; Sorice, M.; Malorni, W.; Garofalo, T.; Matarrese, P. On the role of sphingolipids in cell survival and death. Int. Rev. Cell Mol. Biol. 2020, 351, 149–195.
- Nagahashi, M.; Takabe, K.; Terracina, K.P.; Soma, D.; Hirose, Y.; Kobayashi, T.; Matsuda, Y.; Wakai, T. Sphingosine-1-phosphate transporters as targets for cancer therapy. Biomed. Res. Int. 2014, 2014, 651727.
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell. Mol. Life Sci. 2001, 58, 2053–2068.
- Sassa, T.; Suto, S.; Okayasu, Y.; Kihara, A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta 2012, 1821, 1031–1037.
- Mashhadi Akbar Boojar, M.; Mashhadi Akbar Boojar, M.; Golmohammad, S. Ceramide pathway: A novel approach to cancer chemotherapy. Egypt. J. Basic Appl. Sci. 2018, 5, 237–244.
- Carpinteiro, A.; Dumitru, C.; Schenck, M.; Gulbins, E. Ceramide-induced cell death in malignant cells. Cancer Lett. 2008, 264, 1–10.
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150.
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191.
- Jurowski, K.; Kochan, K.; Walczak, J.; Barańska, M.; Piekoszewski, W.; Buszewski, B. Analytical techniques in lipidomics: State of the art. Crit. Rev. Anal. Chem. 2017, 47, 418–437.
- Serdyuk, I.N.; Zaccai, N.R.; Zaccai, J.; Zaccai, G. Methods in Molecular Biophysics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2017.
- Lee, Y.H.; Tan, C.W.; Venkatratnam, A.; Tan, C.S.; Cui, L.; Loh, S.F.; Griffith, L.; Tannenbaum, S.R.; Chan, J.K.Y. Dysregulated sphingolipid metabolism in endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E1913–E1921.
- Ramos-Garcia, V.; Ten-Doménech, I.; Moreno-Giménez, A.; Gormaz, M.; Parra-Llorca, A.; Shephard, A.P.; Sepúlveda, P.; Pérez-Guaita, D.; Vento, M.; Lendl, B. ATR-FTIR spectroscopy for the routine quality control of exosome isolations. Chemom. Intell. Lab. Syst. 2021, 217, 104401.
- Guleken, Z.; Bulut, H.; Depciuch, J.; Tarhan, N. Diagnosis of endometriosis using endometrioma volume and vibrational spectroscopy with multivariate methods as a noninvasive method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120246.
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004.
- Raghavachari, R. Near-Infrared Applications in Biotechnology; CRC Press: Boca Raton, FL, USA, 2020.
- Barth, A.; Haris, P.I. Biological and Biomedical Infrared Spectroscopy; IOS Press: Amsterdam, The Netherlands, 2009.
- Abidi, N. FTIR Microspectroscopy: Selected Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2021.
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791.
- Sabbatini, S.; Conti, C.; Orilisi, G.; Giorgini, E. Infrared spectroscopy as a new tool for studying single living cells: Is there a niche? Biomed. Spectrosc. Imaging 2017, 6, 85–99.
- Magalhães, S.; Goodfellow, B.J.; Nunes, A. FTIR spectroscopy in biomedical research: How to get the most out of its potential. Appl. Spectrosc. Rev. 2021, 56, 869–907.
- Delfino, I.; Portaccio, M.; Della Ventura, B.; Mita, D.; Lepore, M. Enzyme distribution and secondary structure of sol–gel immobilized glucose oxidase by micro-attenuated total reflection FT-IR spectroscopy. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 304–310.
- Ricciardi, V.; Portaccio, M.; Piccolella, S.; Manti, L.; Pacifico, S.; Lepore, M. Study of SH-SY5Y cancer cell response to treatment with polyphenol extracts using FT-IR spectroscopy. Biosensors 2017, 7, 57.
- Ricciardi, V.; Portaccio, M.; Manti, L.; Lepore, M. An FTIR Microspectroscopy Ratiometric Approach for Monitoring X-ray Irradiation Effects on SH-SY5Y Human Neuroblastoma Cells. Appl. Sci. 2020, 10, 2974.
- d’Apuzzo, F.; Nucci, L.; Delfino, I.; Portaccio, M.; Minervini, G.; Isola, G.; Serino, I.; Camerlingo, C.; Lepore, M. Application of vibrational spectroscopies in the qualitative analysis of gingival crevicular fluid and periodontal ligament during orthodontic tooth movement. J. Clin. Med. 2021, 10, 1405.
- Kallenbach-Thieltges, A.; Großerüschkamp, F.; Mosig, A.; Diem, M.; Tannapfel, A.; Gerwert, K. Immunohistochemistry, histopathology and infrared spectral histopathology of colon cancer tissue sections. J. Biophotonics 2013, 6, 88–100.
- Lasch, P.; Haensch, W.; Naumann, D.; Diem, M. Imaging of colorectal adenocarcinoma using FT-IRmicrospectroscopy and cluster analysis. Biochim. Biophys. Acta 2004, 1688, 176–186.
- Bird, B.; Bedrossian, K.; Laver, N.; Miljković, M.; Romeo, M.J.; Diem, M. Detection of breast micro-metastases in axillary lymph nodes by infrared micro-spectral imaging. Analyst 2009, 134, 1067–1076.
- Ooi, G.J.; Fox, J.; Siu, K.; Lewis, R.; Bambery, K.R.; Mcnaughton, D.; Wood, B.R. Fourier transform infrared imaging and small angle x-ray scattering as a combined biomolecular approach to diagnosis of breast cancer. Med. Phys. 2008, 35, 2151–2161.
- Baker, M.J.; Gazi, E.; Brown, M.D.; Shanks, J.H.; Gardner, P.; Clarke, N.W. FTIR-based spectroscopic analysis in the identification of clinically aggressive prostate cancer. Br. J. Cancer 2008, 99, 1859–1866.
- Baker, M.J.; Gazi, E.; Brown, M.D.; Shanks, J.H.; Clarke, N.W.; Gardner, P. Investigating FTIR based histopathology for the diagnosis of prostate cancer. J. Biophotonics 2009, 2, 104–113.
- Lovergne, L.; Lovergne, J.; Bouzy, P.; Untereiner, V.; Offroy, M.; Garnotel, R.; Thiéfin, G.; Baker, M.J.; Sockalingum, G.D. Investigating pre-analytical requirements for serum and plasma based infrared spectro-diagnostic. J. Biophotonics 2019, 12, e201900177.
- Theakstone, A.G.; Rinaldi, C.; Butler, H.J.; Cameron, J.M.; Confield, L.R.; Rutherford, S.H.; Sala, A.; Sangamnerkar, S.; Baker, M.J. Fourier-transform infrared spectroscopy of biofluids: A practical approach. Transl. Biophotonics 2021, 3, e202000025.
- Bruun, S.W.; Kohler, A.; Adt, I.; Sockalingum, G.D.; Manfait, M.; Martens, H. Correcting attenuated total reflection-Fourier transform infrared spectra for water vapor and carbon dioxide. Appl. Spectrosc. 2006, 60, 1029–1039.
- Vaccari, L.; Birarda, G.; Grenci, G.; Pacor, S.; Businaro, L. Synchrotron radiation infrared microspectroscopy of single living cells in microfluidic devices: Advantages, disadvantages and future perspectives. J. Phys. Conf. Ser. 2012, 359, 012007.
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodrıguez, L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. Trends Anal. Chem. 2009, 28, 263–278.
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodrıguez, L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. Trends Anal. Chem. 2009, 28, 393–403.
- Abdelrazzak, A.B.; Hezma, A.M.; El-Bahy, G.S. ATR-FTIR spectroscopy probing of structural alterations in the cellular membrane of abscopal liver cells. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183726.
- Di Santo, R.; Vaccaro, M.; Romanò, S.; Di Giacinto, F.; Papi, M.; Rapaccini, G.L.; De Spirito, M.; Miele, L.; Basile, U.; Ciasca, G. Machine Learning-Assisted FTIR Analysis of Circulating Extracellular Vesicles for Cancer Liquid Biopsy. J. Pers. Med. 2022, 12, 949.
- Robinson, H.; Molendijk, J.; Shah, A.K.; Rahman, T.; Anderson, G.J.; Hill, M.M. Rapid Assessment of Lipidomics Sample Purity and Quantity Using Fourier-Transform Infrared Spectroscopy. Biomolecules 2022, 12, 1265.
- Schramm, C. High temperature ATR-FTIR characterization of the interaction of polycarboxylic acids and organotrialkoxysilanes with cellulosic material. Biochim. Biophys. Acta 2020, 43, 118815.
- Whyman, R.; Hunt, K.; Page, R.; Rigby, S. A high-pressure spectroscopic cell for FTIR measurements. J. Phys. E Sci. Instr. 2000, 17, 559.
- Reffner, J.A. Advances in Infrared Microspectroscopy and Mapping Molecular Chemical Composition at Submicrometer Spatial Resolution. Spectroscopy 2018, 33, 12–17.
- Beasley, M.M.; Bartelink, E.J.; Taylor, L.; Miller, R.M. Comparison of transmission FTIR, ATR, and DRIFT spectra: Implications for assessment of bone bioapatite diagenesis. J. Archaeol. Sci. 2014, 46, 16–22.
- Horgnies, M.; Chen, J.; Bouillon, C. Overview about the use of Fourier transform infrared spectroscopy to study cementitious materials. WIT Trans. Eng. Sci 2013, 77, 251–262.
- Gendreau, R.M.; Burton, R. The KBr Pellet: A Useful Technique for Obtaining Infrared Spectra of Inorganic Species. Appl. Spectrosc. 1979, 33, 581–584.
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.-M. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim. Biophys. Acta 1999, 1422, 105–185.
- Grdadolnik, J. ATR-FTIR spectroscopy: Its advantage and limitations. Acta Chim. Slov. 2002, 49, 631–642.
- Kazarian, S.; Chan, K. Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim. Biophys. Acta 2006, 1758, 858–867.
- Fringeli, U.P.; Günthard, H.H. Infrared membrane spectroscopy. Mol. Biol. Biochem. Biophys. 1981, 31, 270–332.
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429.
- Yoshida, S.; Koike, K. Lipid and membrane dynamics in biological tissues in nfrared spectroscopic studies. Adv. Planar Lipid Bilayers Liposomes 2011, 13, 1–32.
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179.
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506.
- Derenne, A.; Claessens, T.; Conus, C.; Goormaghtigh, E. Infrared spectroscopy of membrane lipids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1074–1081.
- Derenne, A.; Vandersleyen, O.; Goormaghtigh, E. Lipid quantification method using FTIR spectroscopy applied on cancer cell extracts. Biochim. Biophys. Acta 2014, 1841, 1200–1209.
- Bligh, E.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917.
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643.
- Villalaín, J.; Ortiz, A.; Gómez-Fernández, J.C. Molecular interactions between sphingomyelin and phosphatidylcholine in phospholipid vesicles. Biochim. Biophys. Acta 1988, 941, 55–62.
- Nicolini, C.; Kraineva, J.; Khurana, M.; Periasamy, N.; Funari, S.S.; Winter, R. Temperature and pressure effects on structural and conformational properties of POPC/SM/cholesterol model raft mixtures—A FT-IR, SAXS, DSC, PPC and Laurdan fluorescence spectroscopy study. Biochim. Biophys. Acta 2006, 1758, 248–258.
- Beljebbar, A.; Amharref, N.; Lévèques, A.; Dukic, S.; Venteo, L.; Schneider, L.; Pluot, M.; Manfait, M. Modeling and quantifying biochemical changes in C6 tumor gliomas by Fourier transform infrared imaging. Anal. Chem. 2008, 80, 8406–8415.
- Beljebbar, A.; Dukic, S.; Amharref, N.; Bellefqih, S.; Manfait, M. Monitoring of biochemical changes through the C6 gliomas progression and invasion by Fourier transform infrared (FTIR) imaging. Anal. Chem. 2009, 81, 9247–9256.
- Dreissig, I.; Machill, S.; Salzer, R.; Krafft, C. Quantification of brain lipids by FTIR spectroscopy and partial least squares regression. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 2069–2075.
- Gasper, R.; Dewelle, J.; Kiss, R.; Mijatovic, T.; Goormaghtigh, E. IR spectroscopy as a new tool for evidencing antitumor drug signatures. Biochim. Biophys. Acta 2009, 1788, 1263–1270.
- Gasper, R.; Vandenbussche, G.; Goormaghtigh, E. Ouabain-induced modifications of prostate cancer cell lipidome investigated with mass spectrometry and FTIR spectroscopy. Biochim. Biophys. Acta 2011, 1808, 597–605.
- Mereghetti, P.; Corsetto, P.A.; Cremona, A.; Rizzo, A.M.; Doglia, S.M.; Ami, D. A Fourier transform infrared spectroscopy study of cell membrane domain modifications induced by docosahexaenoic acid. Biochim. Biophys. Acta 2014, 1840, 3115–3122.
- Türker-Kaya, S.; Kına, A. Calorimetric and spectroscopic investigation of the interaction of chemotherapeutic agent carboplatin with sphingomyelin lipids. J. Therm. Anal. Calorim. 2021, 146, 2515–2522.
- Moore, D.J.; Rerek, M.E.; Mendelsohn, R. FTIR spectroscopy studies of the conformational order and phase behavior of ceramides. J. Phys. Chem. B 1997, 101, 8933–8940.
- Corbe, E.; Laugel, C.; Yagoubi, N.; Baillet, A. Role of ceramide structure and its microenvironment on the conformational order of model stratum corneum lipids mixtures: An approach by FTIR spectroscopy. Chem. Phys. Lipids 2007, 146, 67–75.
- De la Arada, I.; González-Ramírez, E.J.; Alonso, A.; Goñi, F.M.; Arrondo, J.-L.R. Exploring polar headgroup interactions between sphingomyelin and ceramide with infrared spectroscopy. Sci. Rep. 2020, 10, 17606.
- López-García, F.; Villaín, J.; Gómez-Fernández, J.C. Effect of sphingosine and stearylamine on the interaction of phosphatidylserine with calcium. A study using DSC, FT-IR and 45Ca2+-binding. Biochim. Biophys. Acta 1995, 1236, 279–288.
- Gómez-Fernández, J.C.; Villalaín, J. The use of FT-IR for quantitative studies of the apparent pKa of lipid carboxyl groups and the dehydration degree of the phosphate group of phospholipids. Chem. Phys. Lipids 1998, 96, 41–52.
- Butler, H.J.; Brennan, P.M.; Cameron, J.M.; Finlayson, D.; Hegarty, M.G.; Jenkinson, M.D.; Palmer, D.S.; Smith, B.R.; Baker, M.J. Development of high-throughput ATR-FTIR technology for rapid triage of brain cancer. Nat. Commun. 2019, 10, 4501.
- De Meutter, J.L.; Goormaghtigh, E. FTIR imaging of protein microarrays for high throughput secondary structure determination. Anal. Chem. 2021, 93, 3733–3741.
- Harrigan, G.G.; LaPlante, R.H.; Cosma, G.N.; Cockerell, G.; Goodacre, R.; Maddox, J.F.; Luyendyk, J.P.; Ganey, P.E.; Roth, R.A. Application of high-throughput Fourier-transform infrared spectroscopy in toxicology studies: Contribution to a study on the development of an animal model for idiosyncratic toxicity. Toxicol. Lett. 2004, 146, 197–205.
- Ellis, D.I.; Dunn, W.B.; Griffin, J.L.; Allwood, J.W.; Goodacre, R. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics 2007, 8, 1243–1266.
- Pachetti, M.; Zupin, L.; Venturin, I.; Mitri, E.; Boscolo, R.; D’amico, F.; Vaccari, L.; Crovella, S.; Ricci, G.; Pascolo, L. FTIR Spectroscopy to Reveal Lipid and Protein Changes Induced on Sperm by Capacitation: Bases for an Improvement of Sample Selection in ART. Int. J. Mol. Sci. 2020, 21, 8659.
More
