Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Barbora Šťastná and Version 2 by Sirius Huang.
The MRE11, RAD50, and NBN genes encode for the nuclear MRN protein complex, which senses the DNA double strand breaks and initiates the DNA repair. The MRN complex also participates in the activation of ATM kinase, which coordinates DNA repair with the p53-dependent cell cycle checkpoint arrest. Carriers of homozygous germline pathogenic variants in the MRN complex genes or compound heterozygotes develop phenotypically distinct rare autosomal recessive syndromes characterized by chromosomal instability and neurological symptoms.
- NBN
- MRE11
- RAD50
- DNA repair
- MRN complex
1. Introduction
Maintenance of the tissue homeostasis relies on intracellular pathways regulating the genome stability, DNA integrity, and appropriate immune surveillance. Although DNA is a chemically stable molecule, its integrity is continually threatened by various endogenous or exogenous processes that alter the structural organization of DNA at different levels, from bases to nucleoprotein assembly of chromatin [1]. Elimination of DNA lesions is mediated by specific DNA repair pathways. Their activation is carefully integrated into a complex intracellular signaling network called the DNA damage response (DDR) [2]. Large number of proteins encoded predominantly by tumor suppressor genes are involved in DNA repair and the DDR.
Most commonly, DNA double-strand breaks (DSB) arise as toxic DNA lesions with the highest tumor-promoting potential in mitotically active cells [3]. In these pathological settings, the DSB result from ionizing radiation exposure, genotoxic DNA impairment caused by various intrinsic processes, or exogenous chemicals that threatened DNA (referred to as clastogens) [4]. The DSB are predominantly repaired by two different pathways, which include homology-directed repair or non-homologous end-joining. DSB repair is initiated by the MRN complex consisting of three highly conserved nuclear proteins, MRE11, RAD50, and NBN. The MRN complex serves as a central hub that senses, processes, and signals DSB and directs the repair strategy to HR or NHEJ through interactions with proteins processing of the broken DNA ends [1][5][6].
2. Structure of the MRN Complex
The MRN complex consists of a symmetrical dimer assembled from two MRE11, RAD50 protomers (M2R2) that are stabilized by NBN protein(s) [7]. The entire complex is a dynamic molecular structure consisting of a globular DNA binding domain and two coiled-coil arms protruding 60 nm apart [8]. Although the detailed molecular structure of the human MRN complex has not yet been solved, its structure should be similar in other species (Figure 1) due to the high conservation of structural and functional features of the MRN complex [9][10][11][12].
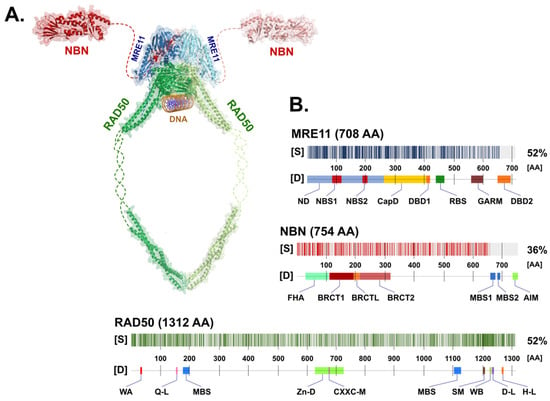
Figure 1. (A) Schematic appearance of the MRN complex interacting with dsDNA. Individual components include NBN (Protein Data Bank (PDB) ID: 3HUE; Schizosaccharomyces pombe), MRE11 (PDB ID: 4FBW; S. pombe), and RAD50 (PDB ID: 5DAC; Chaetomium thermophilum), modelled using PyMol (https://pymol.org/; version 2.5.2). Missing (non-crystallized) parts of the proposed structures for the NBN and RAD50 proteins are indicated by dashed lines. (B) The degree of similarity [S] between human (shown) and non-human paralogs of MRN complex proteins with the determined 3D structure used in panel (A). The positions of protein domains [D] of each MRN protein are shown in the figure. Abbreviations of the protein domains are explained in the text.
2.1. The Nuclease MRE11
The MRE11 gene, localized on chromosome 11q21, codes for a canonical transcript consisting of 20 exons (19 coding; NM_005591). Its protein product, MRE11 nuclease (meiotic recombination 11 homolog; OMIM: *600814), consists of 708 amino acids (aa) forming an 81 kDa nuclear protein (Figure 2) [13].
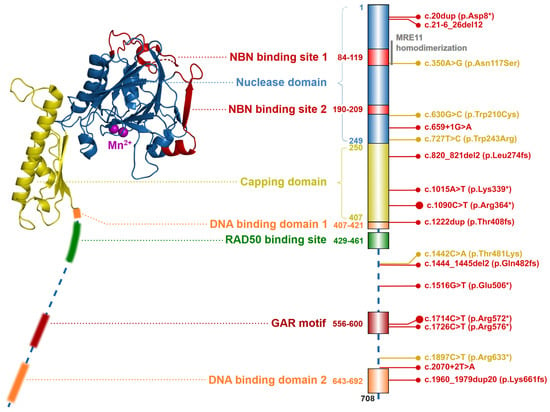
Figure 2. Schematic structure of the MRE11 protein (left; from S. pombe, PDB ID: 4FBQ). Two Mn2+ ions are shown as purple spheres in the center of the nuclease domain. The structure of flexible C-terminal parts (indicated by dashed line) containing the DNA binding domains, GAR motif, and RAD50 binding site were not resolved yet. Colors of protein domains in structure model (left) corresponds to that in schematic bar chart representing domain composition and the most frequent germline loss-of-function variants with allele frequency >10−5 in the GnomAD database (the size of the lollipop reflects the frequency of a variant). Yellow lollipops correspond to variants causing Ataxia-telangiectasia-like disorder. Grey notes highlight the important domain interactions.
Two MRE11 proteins homodimerize through their N-terminal nuclease domains (ND; aa residues 1–249). Each ND includes two NBN binding sites (NBS1; aa residues 84–119 and NBS2; aa residues 190–209) and five conserved phosphodiesterase motifs, that consist of five histidine residues (His22, His63, His129, His217, His245, His247), two aspartic acid residues (Asp20, Asp60) and an asparagine (Asn128), forming together a nuclease active site. The nuclease active site is bound by two Mn2+ ions which are essential for the ssDNA endonuclease and dsDNA exonuclease activities of MRE11. The first Mn2+ ion is bound to Asp20, His22, Asp60 and His247 and the second Mn2+ ion is coordinated to Asp60, Asn128, His217 and His245 [14][15][16][17]. The adjacent capping domain (CapD; aa residues 230–406) helps to discriminate between ssDNA and dsDNA substrates and controls their correct entry into the nuclease active site [9][14][18]. Two DNA binding domains (DBD1; aa residues 407–421 and DBD2; aa residues 643–692) are located downstream of CapD [7][14][18]. The RAD50 binding site (RBS; aa residues 429–461) is localized between the DBD, and interact with the coiled-coil domain of RAD50. The conserved glycine-arginine-rich motif (GAR motif; amino acid residues 566–600) is methylated by protein arginine methyltransferase 1 (PRMT1), at the MRE11 residues Arg570, Arg572, Arg574, Arg576, Arg577, Arg580 [19][20]. This methylation allows sensitive regulation of MRE11 nuclease activity but does not affect the MRN complex assembly.
The MRE11 protein forms a binding clamp that tethers RAD50 and NBN, which otherwise cannot interact directly [1]. For the MRN complex assembly, each MRE11 molecule first binds to a single RAD50 protein. Subsequently, the MRE11-RAD50 dimer homodimerizes to form a MRE112RAD502 core, bridged and interlocked by two NBN subunits (Figure 1) [9][21].
2.2. The RAD50 ATPase
The RAD50 gene, located on chromosome 5q31.1, encodes for a primary transcript consisting of 25 exons (NM_005732). Its protein product, the RAD50 ATPase (OMIM: *604040) consists of 1312 amino acids (with a molecular mass of 150 kDa) and forms the largest component of the MRN complex.
The entire RAD50 polypeptide chain is folded in half to form a fibrillar structure with antiparallel helices that have a Zn2+-hook on one side (formed by the central portion of the polypeptide) and a globular head containing the catalytic ATPase domain (assembled from N- and C-terminal ends of the polypeptide) on the other one (Figure 3). A similar structural assembly is found in other RAD50 homologs belonging to the structural maintenance of chromosomes (SMC) protein family [22].
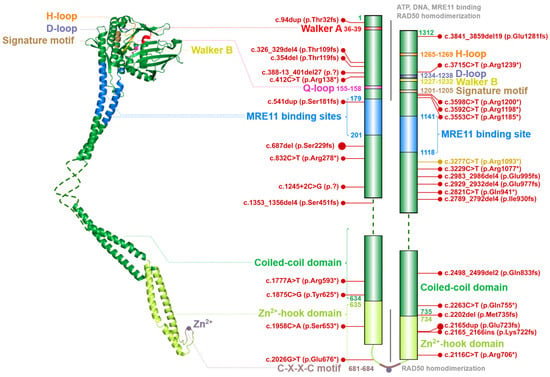
Figure 3. Schematic structure of the RAD50 monomer (left) modelled from globular part of Ch. thermophilum protein (PDB ID: 5DAC) and human coiled-coil domain (PDB ID: 5GOX). Dashed line denotes the unstructured non-crystallized parts. Colors of protein domains in structure model (left) correspond to that in schematic bar chart that summarize the domain composition and the most frequent germline loss-of-function variants with allele frequency >10−5 in the GnomAD database (in red, the size of the lollipops reflects the frequency of a variant). Yellow lollipop corresponds to variant causing Nijmegen breakage syndrome-like disorder. Grey notes highlight important domain interactions.
The ATPase domain consists of six conserved structures, including the Walker A (WA; aa residues 36–39) and Walker B (WB; aa residues 1227–1232), Q-loop (Q-L; aa residues 155–158), signature motif (SM; aa residues 1201–1205), D-loop (D-L; aa residues 1234–1238) and H-loop (H-L; aa residues 1265–1269). These conserved nucleotide-binding motifs are required for interactions with ATP and MRE11 and for the DNA binding [5][14][15][23][24].
A flexible arm, forming an antiparallel coiled-coil domain localized between MRE11 binding sites, is tipped by a hook domain containing a conserved CXXC motif that includes two invariant cysteine residues (Cys681 and Cys684) separated by two hydrophobic amino acids (X). The CXXC motif binds a Zn2+ ion and forms the hook domain mediating RAD50 homodimerization controlled by the ATP binding [7][25][26]. The homodimerization by Zn2+-hook domain include two RAD50 molecules from the same MRN complex and form a predominantly ring-shaped form of MRN complex (Figure 1). However, the interaction of Zn2+-hook domains may also form an intercomplex that is required for long-range tethering of two DNA molecules, such as the resected DNA and its sister chromatid during homologous recombination [5][27][28].
The ATP-dependent RAD50 conformation changes regulate the nuclease activity of MRE11. Once ATP is bound within the globular head of each RAD50 monomer, the RAD50 molecules reach a rigid ‘closed’ conformation and their head domains interact with each other to form a groove appropriate for the accommodation of dsDNA [29][30]. In the ATP-bound, closed state, RAD50 blocks access of dsDNA to MRE11 active site and prevents its nuclease activity. After ATP hydrolysis which leads to large conformational change of RAD50, dsDNA is accessible to the nuclease cleavage by MRE11 [31][32]. Thus, hydrolysis of ATP by RAD50 renders RAD50-MRE11 dimer to the ‘open’ conformation with high processivity of MRE11 exonuclease and endonuclease activities.
2.3. NBN, a Dynamic Connector
The NBN gene, localized on chromosome 5q31.1, codes for a canonical transcript consisting of 16 exons (NM_002485). Its protein product, NBN protein (Nijmegen breakage syndrome protein 1; OMIM: *602667; also known as nibrin or NBS1) consists of 754 amino acids (with molecular mass 85 kDa) [33]. The NBN protein acts as a phosphoprotein-binding and adapter subunit of eukaryotic MRN complexes providing the MRE112RAD502 tetramer with a versatile connector to various signaling or DSB repair proteins [14].
Its N-terminal part contains a forkhead-associated domain (FHA; aa residues 20–108) and two BRCA1 C-terminal domains (BRCT1; aa residues 111–197 and BRCT2; aa residues 219–327) separated by the BRCT linker (BRCTL; aa residues 198–218; Figure 4). FHA and BRCT domains bind multiple phosphorylated proteins regulating the MRN complex interactions. Through an FHA domain, NBN binds the C-terminal-binding protein interacting protein (CtIP; aka retinoblastoma-binding protein 8-RBBP8) [34]. FHA together with BRCT1/2 domains interacts with a phosphorylated mediator of the DNA damage checkpoint 1 (MDC1), which promotes recruitment of repair proteins to the sites of DNA breaks [35][36]. The rest of NBN polypeptide represents a largely unstructured region except for two MRE11 binding sites (MBS1; aa residues 640–662 and MBS2; aa residues 681–692) and ATM interaction motif (AIM; aa residues 734–754), through which NBN recruits ATM to the proximity of DSB. In turn, ATM phosphorylates multiple proteins at chromatin including γ-H2AX (H2A histone family member X) and MRN complex itself, including Ser278 and Ser343 residues of NBN [29][7][25][37].
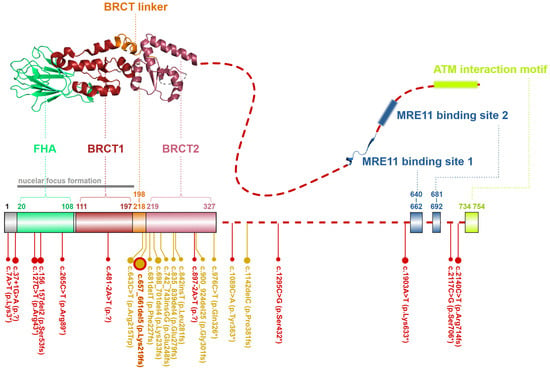
Figure 4. Schematic structure of the NBN protein from S. pombe (up, PDB ID: 3HUE). A dashed line denotes the unstructured non-crystallized parts. Colors of protein domains in structure model (up) correspond to that in schematic bar chart summarizing domain positions and the most frequent germline loss-of-function variants with allele frequency >10−5 in the GnomAD database (the size of the lollipops reflects the frequency of a variant). Yellow lollipops correspond to variants causing Nijmegen breakage syndrome. Grey notes highlight the important domain interaction.
Folding of the MRN complex in the cytosol is facilitated by a chaperon R2TP complex (consisting of PIH1 domain-containing protein 1 (PIH1D1), RNA polymerase II-associated protein 3 (RPAP3) and RUVB-like AAA ATPase 1 and 2 (RUVBL1 and RUVBL2)) [38]. Subsequently, the nuclear localization signal of NBN promotes translocation of the MRN to the nucleus [39]. By direct binding to MRE11, NBN stabilizes the MRN complex and stimulates the MRE11 nuclease activity [38]. Moreover, MRE11-NBN interaction is required for genome integrity and tumor suppression [40]. An important interacting partner of NBN is CtIP protein, a fourth eukaryotic, non-catalytic MRN complex subunit, which is essential for the initiation of DNA ends resection. The CtIP is retained at the break site by phosphorylation-dependent binding to the FHA and BRCT domains of the NBN [21][41][42].
While MRE11 and RAD50 homologs and their M2R2 complexes are ubiquitous across living organisms, the NBN protein (or its homolog XRS2 in Saccharomyces cerevisiae) is a characteristic component of eukaryotic cells only. Recent cryoelectron microscopy analysis of the eukaryotic MRN complex from Ch. thermophilum by Rotheneder and colleagues described a global architecture, revealing a rod-like assembly of RAD50 dimers protruding with their CC domains 60 nm apart from a complex of RAD50 globular head with MRE11 homodimer stabilized by an asymmetrically-bound single NBN molecule [8]. Despite that this work shed light on the possible composition of the human MRN complex, many important questions remained unanswered, including its dynamics during the DSB repair or the composition of the NBN subunit and its interaction with binding partners, including ATM or CtIP [43].
References
- Qiu, S.; Huang, J. MRN complex is an essential effector of DNA damage repair. J. Zhejiang Univ. B 2021, 22, 31–37.
- Benada, J.; Macurek, L. Targeting the Checkpoint to Kill Cancer Cells. Biomolecules 2015, 5, 1912–1937.
- van den Bosch, M.; Bree, R.T.; Lowndes, N.F. The MRN complex: Coordinating and mediating the response to broken chromosomes. EMBO Rep. 2003, 4, 844–849.
- Libri, A.; Marton, T.; Deriano, L. The (Lack of) DNA Double-Strand Break Repair Pathway Choice during V(D)J Recombination. Front. Genet. 2022, 12, 1–10.
- Lamarche, B.J.; Orazio, N.I.; Weitzman, M.D. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 2010, 584, 3682–3695.
- Paull, T.T. 20 Years of Mre11 Biology: No End in Sight. Mol. Cell 2018, 71, 419–427.
- Bian, L.; Meng, Y.; Zhang, M.; Li, D. MRE11-RAD50-NBS1 complex alterations and DNA damage response: Implications for cancer treatment. Mol. Cancer 2019, 18, 169.
- Rotheneder, M.; Stakyte, K.; van de Logt, E.; Bartho, J.D.; Lammens, K.; Fan, Y.; Alt, A.; Kessler, B.; Jung, C.; Roos, W.P.; et al. Cryo-EM structure of the Mre11-Rad50-Nbs1 complex reveals the molecular mechanism of scaffolding functions. Mol. Cell 2023, 83, 167–185.e9.
- Schiller, C.B.; Lammens, K.; Guerini, I.; Coordes, B.; Feldmann, H.; Schlauderer, F.; Möckel, C.; Schele, A.; Strässer, K.; Jackson, S.P.; et al. Structure of Mre11–Nbs1 complex yields insights into ataxia-telangiectasia–like disease mutations and DNA damage signaling. Nat. Struct. Mol. Biol. 2012, 19, 693–700.
- Schiller, C.B.; Seifert, F.U.; Linke-Winnebeck, C.; Hopfner, K.-P. Structural Studies of DNA End Detection and Resection in Homologous Recombination. Cold Spring Harb. Perspect. Biol. 2014, 6, a017962.
- Williams, R.S.; Dodson, G.E.; Limbo, O.; Yamada, Y.; Williams, J.S.; Guenther, G.; Classen, S.; Glover, J.M.; Iwasaki, H.; Russell, P.; et al. Nbs1 Flexibly Tethers Ctp1 and Mre11-Rad50 to Coordinate DNA Double-Strand Break Processing and Repair. Cell 2009, 139, 87–99.
- Lim, H.S.; Kim, J.S.; Park, Y.B.; Gwon, G.S.; Cho, Y. Crystal structure of the Mre11-Rad50-ATPγS complex: Understanding the interplay between Mre11 and Rad50. Genes Dev. 2011, 25, 1091–1104.
- Lee, K.C.; Padget, K.; Curtis, H.; Cowell, I.; Moiani, D.; Sondka, Z.; Morris, N.; Jackson, G.H.; Cockell, S.; Tainer, J.; et al. MRE11 facilitates the removal of human topoisomerase II complexes from genomic DNA. Biol. Open 2012, 1, 863–873.
- Lafrance-Vanasse, J.; Williams, G.J.; Tainer, J.A. Envisioning the dynamics and flexibility of Mre11-Rad50-Nbs1 complex to decipher its roles in DNA replication and repair. Prog. Biophys. Mol. Biol. 2015, 117, 182–193.
- Hopfner, K.-P.; Karcher, A.; Craig, L.; Woo, T.T.; Carney, J.P.; Tainer, J.A. Structural Biochemistry and Interaction Architecture of the DNA Double-Strand Break Repair Mre11 Nuclease and Rad50-ATPase. Cell 2001, 105, 473–485.
- Sacho, E.J.; Maizels, N. DNA repair factor MRE11/RAD50 cleaves 3′-phosphotyrosyl bonds and resects DNA to repair damage caused by topoisomerase 1 poisons. J. Biol. Chem. 2011, 286, 44945–44951.
- Park, Y.B.; Chae, J.; Kim, Y.C.; Cho, Y. Crystal Structure of Human Mre11: Understanding Tumorigenic Mutations. Structure 2011, 19, 1591–1602.
- Williams, R.S.; Moncalian, G.; Williams, J.S.; Yamada, Y.; Limbo, O.; Shin, D.S.; Groocock, L.M.; Cahill, D.; Hitomi, C.; Guenther, G.; et al. Mre11 Dimers Coordinate DNA End Bridging and Nuclease Processing in Double-Strand-Break Repair. Cell 2008, 135, 97–109.
- Lu, R.; Zhang, H.; Jiang, Y.-N.; Wang, Z.-Q.; Sun, L.; Zhou, Z.-W. Post-Translational Modification of MRE11: Its Implication in DDR and Diseases. Genes 2021, 12, 1158.
- Déry, U.; Coulombe, Y.; Rodrigue, A.; Stasiak, A.; Richard, S.; Masson, J.-Y. A Glycine-Arginine Domain in Control of the Human MRE11 DNA Repair Protein. Mol. Cell. Biol. 2008, 28, 3058–3069.
- Gobbini, E.; Cassani, C.; Villa, M.; Bonetti, D.; Longhese, M.P. Functions and regulation of the MRX complex at DNA double-strand breaks. Microb. Cell 2016, 3, 329–337.
- Zabolotnaya, E.; Mela, I.; Henderson, R.M.; Robinson, N.P. Turning the Mre11/Rad50 DNA repair complex on its head: Lessons from SMC protein hinges, dynamic coiled-coil movements and DNA loop-extrusion? Biochem. Soc. Trans. 2020, 48, 2359–2376.
- Rojowska, A.; Lammens, K.; Seifert, F.U.; Direnberger, C.; Feldmann, H.; Hopfner, K. Structure of the Rad50 DNA double-strand break repair protein in complex with DNA. EMBO J. 2014, 33, 2847–2859.
- Moncalian, G.; Lengsfeld, B.; Bhaskara, V.; Hopfner, K.-P.; Karcher, A.; Alden, E.; Tainer, J.A.; Paull, T.T. The Rad50 Signature Motif: Essential to ATP Binding and Biological Function. J. Mol. Biol. 2004, 335, 937–951.
- Hopfner, K.-P.; Craig, L.; Moncalian, G.; Zinkel, R.A.; Usui, T.; Owen, B.A.L.; Karcher, A.; Henderson, B.; Bodmer, J.-L.; McMurray, C.T.; et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 2002, 418, 562–566.
- Remali, J.; Aizat, W.M.; Ng, C.L.; Lim, Y.C.; Mohamed-Hussein, Z.-A.; Fazry, S. In silico analysis on the functional and structural impact of Rad50 mutations involved in DNA strand break repair. PeerJ 2020, 8, e9197.
- Moreno-Herrero, F.; de Jager, M.; Dekker, N.H.; Kanaar, R.; Wyman, C.; Dekker, C. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature 2005, 437, 440–443.
- Park, Y.B.; Hohl, M.; Padjasek, M.; Jeong, E.; Jin, K.S.; Krezel, A.; Petrini, M.H.J.H.J.; Cho, Y.B.P.E.J.Y. Eukaryotic Rad50 functions as a rod-shaped dimer. Nat. Struct. Mol. Biol. 2017, 24, 248–257.
- Syed, A.; A Tainer, J. The MRE11–RAD50–NBS1 Complex Conducts the Orchestration of Damage Signaling and Outcomes to Stress in DNA Replication and Repair. Annu. Rev. Biochem. 2018, 87, 263–294.
- Seifert, F.U.; Lammens, K.; Stoehr, G.; Kessler, B.; Hopfner, K. Structural mechanism of ATP-dependent DNA binding and DNA end bridging by eukaryotic Rad50. EMBO J. 2016, 35, 759–772.
- Käshammer, L.; Saathoff, J.-H.; Lammens, K.; Gut, F.; Bartho, J.; Alt, A.; Kessler, B.; Hopfner, K.-P. Mechanism of DNA End Sensing and Processing by the Mre11-Rad50 Complex. Mol. Cell 2019, 76, 382–394.e6.
- Cejka, P.; Symington, L.S. DNA End Resection: Mechanism and Control. Annu. Rev. Genet. 2021, 55, 285–307.
- Varon, R.; Vissinga, C.; Platzer, M.; Cerosaletti, K.M.; Chrzanowska, K.H.; Saar, K.; Beckmann, G.; Seemanová, E.; Cooper, P.R.; Nowak, N.J.; et al. Nibrin, a Novel DNA Double-Strand Break Repair Protein, Is Mutated in Nijmegen Breakage Syndrome. Cell 1998, 93, 467–476.
- Cilli, D.; Mirasole, C.; Pennisi, R.; Pallotta, V.; D′Alessandro, A.; Antoccia, A.; Zolla, L.; Ascenzi, P.; di Masi, A. Identification of the Interactors of Human Nibrin (NBN) and of Its 26 kDa and 70 kDa Fragments Arising from the NBN 657del5 Founder Mutation. PLoS ONE 2014, 9, e114651.
- Chapman, J.R.; Jackson, S.P. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008, 9, 795–801.
- Stewart, G.S.; Wang, B.; Bignell, C.R.; Taylor, A.M.R.; Elledge, S.J. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 2003, 421, 961–966.
- Iijima, K.; Ohara, M.; Seki, R.; Tauchi, H. Dancing on damaged chromatin: Functions of ATM and the RAD50/MRE11/NBS1 complex in cellular responses to DNA damage. J. Radiat. Res. 2008, 49, 451–464.
- von Morgen, P.; Burdova, K.; Flower, T.G.; O′Reilly, N.J.; Boulton, S.J.; Smerdon, S.J.; Macurek, L.; Hořejší, Z. MRE11 stability is regulated by CK2-dependent interaction with R2TP complex. Oncogene 2017, 36, 4943–4950.
- Carney, J.P.; Maser, R.S.; Olivares, H.; Davis, E.M.; Le Beau, M.; Yates, J.R., 3rd; Hays, L.; Morgan, W.F.; Petrini, J.H. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: Linkage of double-strand break repair to the cellular DNA damage response. Cell 1998, 93, 477–486.
- Kim, J.H.; Grosbart, M.; Anand, R.; Wyman, C.; Cejka, P.; Petrini, J.H. The Mre11-Nbs1 Interface Is Essential for Viability and Tumor Suppression. Cell Rep. 2017, 18, 496–507.
- Anand, R.; Ranjha, L.; Cannavo, E.; Cejka, P. Phosphorylated CtIP Functions as a Co-factor of the MRE11-RAD50-NBS1 Endonuclease in DNA End Resection. Mol. Cell 2016, 64, 940–950.
- Zhang, T.; Zhou, Z.; Yang, H.; Wang, W. MRE11-RAD50-NBS1-CtIP: One key nuclease ensemble functions in the maintenance of genome stability. Genome Instab. Dis. 2022, 3, 123–135.
- Wojtaszek, J.L.; Williams, R.S. The ends in sight: Mre11-Rad50-Nbs1 complex structures come into focus. Mol. Cell 2023, 83, 160–162.
More
