Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zarini Ismail and Version 2 by Sirius Huang.
Aerogel is one of the most interesting materials globally. The network of aerogel consists of pores with nanometer widths, which leads to a variety of functional properties and broad applications. Aerogel is categorized as inorganic, organic, carbon, and biopolymers, and can be modified by the addition of advanced materials and nanofillers.
- aerogel
- silica
- biopolymer
- biomedical application
- wound healing
- drug delivery
1. Introduction
Aerogel is a nanostructured material that is gaining popularity as a structural alternative for insulation in a variety of uses, ranging from residences and commercial structures to offshore platforms and spacecraft. Aerogel insulator is thought to provide 40 times the shielding effect of fiber glass, allowing it to be used in space-constrained applications. It is a low-density, high dielectric strength, high specific surface areas, low thermal conductivities, and extremely porous foam with interconnected nanostructures [1][2][1,2]. Aerogel is composed of approximately 99.8 percent space, giving it a spectral look, and garnering the name of ‘solid smoke’ [3]. It is typically composed of silica and may take numerous shapes. However, organic polymers, inorganic, carbon allotropes, polysaccharides, transition metals, and nanostructures of semiconductors may also synthesize aerogels [4]. Aerogel is created by drying gels at extremely elevated heat.
In the early 1930s, Kistler and Learned invented the first aerogel by supercritical drying a wet gel and extracting the liquid [5]. It was employed as a tobacco filler and thickener, whereas silica aerogel was used as a thermal insulating blanket. Despite the numerous benefits that silica as well as other inorganic compounds can bring in the production of aerogel, conventional aerogel raw resources are still derived from petrochemical sources. On the other hand, the difficult multistage preparation method stymied the development of aerogel. Nonetheless, native aerogel with a single element is typically afflicted by serious issues such as weak mechanical properties, and a lack of functionalities. The name “aerogel” resurfaced in the 1970s, with the rising use of sol–gel synthesis processes and the usage of aerogel to store rocket fuels [6]. Following that, important efforts were made to simplify the synthesis methods, particularly drying to achieve a low-cost and simple synthesis of aerogel. This paved the way for a wide range of aerogel to be used in various fields of application due to their open structure and lightweight [5][7][8][5,7,8]. To improve aerogel performance, significant growth in the emergence of future aerogel with varied physicochemical features and functional abilities is required [9][10][9,10]. For example, aerogel-based biomaterials are now made from a variety of sources or components that imitate the structure of a biological extracellular matrix. The tissues that surround this structure serve as support cells and are affected biochemically by it. Even though an aerogel network has also hybridized with a wide variety of nanostructures and improved functional properties such as antifungal or antimicrobial performance.
2. Type of Aerogels and Properties
Different varieties of aerogels were produced during the last few decades as the methods for the synthesis and drying of aerogels improved. They can be classified as inorganic aerogels (silica, alumina, and titania), polymer-based, carbon allotropes (nanotubes and graphene), and natural macromolecule-based aerogels (alginate, starch, gelatin, protein, nanocellulose and chitosan) [11][12][13,14]. Typically, silica-based aerogels are the most potential candidate materials owing to their distinctive characteristics, such as low thermal conductivity (15–20 W/mK), low density (0.003–0.5 g/cm3), and large surface area [13][15]. They are generally fragile, have poor mechanical properties, and require a lengthy processing technique, hence limiting their application range [10]. Many attempts to increase the quality of silica-based aerogels have already been made, including using (i) adaptable silica catalysts in the strand, (ii) enhanced polymer cross-linking, (iii) accelerated ageing processes in different solutions, (iv) adding nanofillers, and (v) polymerizing the precursor in advance of gelation. For example, it has been shown that the combination of silica with methacrylate polymer to improve the polymerization resulted in enhanced mechanical performance and other parameters, including densities, areas, pore diameters, and void content [14][16]. Silica aerogels through polymer modification are illustrated in Figure 1. They are classified as silica aerogels reinforced polymer, fabricated via cross-linked via water-oil aqueous solution in high-internal stage emulsion substance. This novel material shows a superior performance property over pure silica aerogels [15][17]. In addition, Posada et al. produced ceria-containing silica aerogel via a three-way catalyst approach in incorporation with a new rapid supercritical separation method. They employed a polyether to strengthen the aging process and accelerate the gelation time [16][18]. This innovative technique can reduce the time taken to prepare wet gels, including gelation, ageing, and solvent exchange from days to seconds [17][19].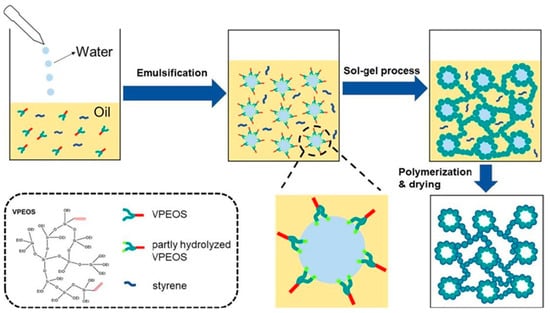
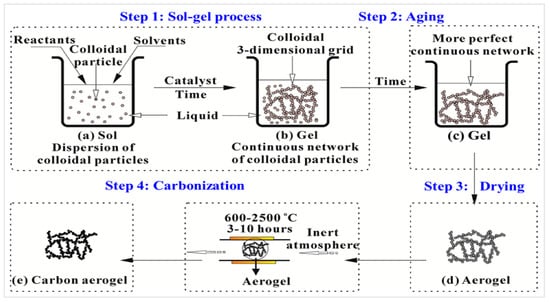
Table 1.
Different types of aerogels with their respective properties.
| Types | Main Component |
Properties | Weakness | Methods for Improvement |
Applications | References |
|---|---|---|---|---|---|---|
| Silica | Tetraethylorthosilicate (TEOS) and methyltrimethoxysilane (MTMS) |
Low heat conductivity, large built-up area, low density |
Fragile, have poor mechanical properties and require a lengthy processing technique |
Use precursors in the backbone, surface-crosslinking with a polymer, prolonged aging incorporating, polymerizing |
Photocatalysts, Thermal insulation, absorbent pollutants |
[13][15[41],43] |
| Polymer | Cellulose/ conducting polymer |
High moduli and fatigue resistance |
Monolithic, prone to defects, length processing and costly |
Usage of synthetic polymer | Additives (foods, cosmetics) construction, materials, drug delivery carrier | [28][42][30,44] |
][81]. A nano-porous silica aerogel was developed for drug delivery for oral administration of paclitaxel (PTX), an anticancer drug. The excellent biocompatibility of this aerogel was proven by the reduced side effects of drug and inhibited tumour growth [59][82]. Furthermore, silica–gelatin hybrid aerogel has a potential for local and non-invasive drug delivery because they are biodegradable and biocompatible within tissue cells [60][83]. In addition, aerogels produce from marine polymer such as chitosan exhibit a potential prospect in wound healing due to their antimicrobial activity. Piatkowski et al. developed a new chitosan-based aerogel with enhanced properties to improve the healing of burn wounds. The studies demonstrated that the proposed chitosan aerogel containing Au nanoparticles were biocompatible and promoted fibroblast proliferation [61][84]. Batista et al. developed a hybrid alginate-chitosan aerogel fibre and assess their effect in wound healing application using the emulsion gelation method. In vitro model assessment revealed that they were non-cytotoxic and promoted wound healing [62][85]. In another study, the hybrid chitosan–alginate aerogel microparticles were also prepared using the emulsion gelation technique. The toxicity test showed that the alginate-chitosan carrier induced moderate lung inflammation along with some damage to kidneys and liver [63][86]. However, conventionally prepared chitosan aerogel exhibited a number of defects, including low porosity, an irregular structure, and an easiness to deform, which limited their biocompatibility [64][87].
Beside chitosan, alginate is another biomaterial that has been intensively researched in biomedical fields. For example, Franco et al. used mesoglycan (MSG) and impregnated calcium alginate aerogel (CAA) to treat a wound. Both human keratinocytes and fibroblasts were resistant to the cytotoxic effects of MSG on CAA, as shown by an in vitro experiment [65][88]. In other applications, aerogel microspheres based on alginate and hyaluronic acid demonstrate high porosity and good in vitro aerodynamic properties [66][89]. Carbon-based aerogels are unique since it consists of networks of 3D nanostructures with a high volume of air-filled nano-porous, high porosity, low density, and a large surface area [67][90]. These properties endow aerogels with a rapid response signal, high selectivity, and super sensitivity for sensing a variety of biomedical targets. The synthesis of carbon-aerogel scaffolds containing biocompatible ceramic nanoparticles of tricalcium phosphate has been disclosed by Tevlek et al. Due to their high gelatine content and highly porous structure, the materials exhibited good biocompatibility and supported cell growth for 14 days [68][91].
Table 2.
Summary of studies on compatibility of aerogels for biomedical applications.
| Aerogels | Method | Remarks | References | Year | ||
|---|---|---|---|---|---|---|
| 1 | Cellulose | Freeze drying and polymerization |
Higher biocompatible with catalase immobilization |
[52][75] | 2019 | |
| 2 | Silica | Freeze-drying | Biocompatible for drug carrier |
[59][82] | 2019 | |
| Carbon | Carbon/CNT/ graphene |
High specific surface area and porosity, low density, good electrical conductor, good chemical stability, and hydrophobicity, |
Low electrical conductivities and reduced heat transmission via the aerogel backbone phase with related organic precursor | Focused on carbon aerogel-based biomass |
Electrodes, in supercapacitors, adsorbents for phenol |
[37][43][39,45] |
| Inorganic | Oxide/ metallic/ chalcogenide | Ultra-high surface area and high open porosity |
High production cost | Hybrid aerogel formation |
Energy conversion, storage application |
[44][45][46,47] |
| Organic | Biopolymer | High compressive strength, high surface area |
Poor mechanical properties | Incorporated with inorganic fillers | Biosensor, Medical implantable device. |
[46][48] |
3. Biomedical Applications of Aerogel
Aerogel is an appealing substance for the biomedical field due to their distinctive properties, which include low density, porous structure, extensive surface area, and high strength. It is also versatile in terms of sol–gel biochemistry. Aerogel typically used in biomedicine to encapsulate bioactive compounds with low solubility or stability as well as to create artificial scaffolds for tissue engineering and materials for chronic wound dressings. Other than that, many studies also discussed the applications of aerogel, such as for drug delivery carriers, anti-toxicity, and antioxidants. Although the technology and composition of aerogels are varied, the aerogels applied in the biological system must be made of biocompatible, and preferably biodegradable material.
Biocompatibility is the ability of materials to be functional in a biological system without causing harm. Biocompatibility must be determined before any substance can be used in biological applications. Table 2 summarises biocompatibility testing of various materials using in vitro and in vivo methods. Bajpai et al. studied the biocompatibility of 3D-structure graphene aerogel (GA). This 3D, ultra-lightweight and hydrophobic GA was produced by the one-step pyrolysis of sugar and ammonium chloride. GA showed excellent adsorption capacity for various biogenic amines, bacteria contaminants such as Staphylococcus aureus, and other toxins, especially in food safety applications. The biocompatibility of the synthesised GA is also determined by cell proliferation efficiency and wound healing ability [47][70]. In another work, Liu et al. created an extremely porous aerogel consisting of graphene oxide (GO) and Type I collagen (COL) using the sol–gel approach. This study demonstrated that 0.1% GO-COL aerogel had good biocompatibility in vivo, making it a potential scaffold to support bone regeneration and tissue engineering [48][71]. Zhou et al. found that the combination of hydroxyapatite and graphene to produce a new aerogel improved microbial electrocatalysis due to its higher interfacial and biocompatibility for bacterial growth [49][72].
Other biomaterials, such as bacterial cellulose (BC) also had excellent biocompatibility and had a low immunogenic potential [50][73]. BC aerogel is known for being fragile, very light, open-pored and transversally isotropic materials for various biomedical applications. Salehi et al. put clay nanoplatelets over the BC membrane to form a nano-fibrillated template for aniline in situ polymerization, creating a double linked network of electrically conductive pathways in the aerogel. Clay and polyaniline had a synergistic effect on biocompatibility and cell adhesion, with no mutagenic or carcinogenic effects [51][74]. In another study, a novel biocompatible BC aerogel modified with poly (glycidyl methacrylate) (PGMA) was fabricated. The incorporation of PGMA and BC aerogel improved its biocompatibility following the immobilisation of catalase [52][75]. BC aerogel had the highest modulus, porosity, and specific surface area among cellulose aerogels. Even so, the production of BC was hindered by a lengthy production period, a low yield, and a high price, which diminished interest in its further clinical applications.
Another biomaterial with exceptional properties and being more biocompatible within cells is silica aerogel. Their main limitations however, are fragility and high hygroscopicity [53][76]. In a study by Lazar et al., silica aerogel was hybridised with industrial manufactured bovine casein, using tetramethyl orthosilicate (TMOS) and co-gelation. The CHO-K1 Chinese hamster ovary cell line was used to test the in vitro biocompatibility of hybrid aerogel. It has been demonstrated that silica-casein aerogel are highly biocompatible and, to all intents and purposes, non-toxic to CHO-K1 cells [54][77]. According to the findings by Sani et al., the hydroxyapatite (HA)-mixed with silica aerogel with a weight ratio of 0.5 had the highest bioactivity and biocompatibility [55][78]. Resveratrol has been thought to help with or even cure osteoarthritis. Qin et al. synthesized a resveratrol-loaded silica aerogel (RSA) using the sol–gel method and exploited it as a drug delivery vehicle. According to the results of the study, RSA is inexpensive, biocompatible, and has relatively high loading rate of 19%. Initial in vitro toxicity testing revealed that RSA is biocompatible stable, and may be used to treat osteoarthritis due to its anti-inflammatory effects [56][79]. In another report, nanofibrous silica hybrid aerogel was biocompatible to healthy cells but their antitumour activity significantly increased when loaded with camptothecin (CPT) [57][80]. Kiraly et al. in their study injected a fluorescein-labeled silica-gelatin aerogel microparticles (FSGM) into the peritoneum of mice to assess acute toxicity. They reported no physiological abnormalities or disorder were discovered after a three-week-long experiment [58
| 3 | |||||
| Graphene | oxide- collagen |
Sol–gel process | 0.1% GO-COL aerogel presented reliable biocompatibility |
[48][71] | 2019 |
| 4 | Graphene | Pyrolysis | Cell viability was observed even at high concentrations |
[47][70] | 2019 |
| 5 | Chitin | Supercritical CO2 drying and freeze-drying | Good biocompatibility (cell viability >90% | [69][92] | 2019 |
| 6 | Alginate- chitosan |
Supercritical drying of CO2 | Cell viability values >70 % | [62][85] | 2020 |
| 7 | Alginate- Chitosan |
Emulsion gelation | Resulted in mild lung- congestion |
[63][86] | 2020 |
| 8 | Silica | Aqueous sol–gel ambient pressure drying | Not toxic to normal human osteoblast cell line |
[55][78] | 2020 |
| 9 | Silica | Co-gelation in the sol–gel, supercritical CO2 |
Highly biocompatible and practically inert towards CHO-K1 cells |
[54][77] | 2020 |
| 10 | Silica | Sol–gel | Good biocompatibility | [56][79] | 2020 |
| 11 | Silica | Freeze-drying and cross- linking | Excellent biocompatibility to human cells | [57][80] | 2020 |
| 12 | Carbon | Freeze-drying | Cells able to adapt to microenvironment and able for growth |
[68][91] | 2020 |
| 13 | Composite | Freeze-drying | Good biocompatibility of mouse lung fibroblasts (L929) cells on the membrane |
[51][74] | 2021 |
| 14 | Silica | Sol–gel combined with co-gelation |
All mice were healthy after being injected with aerogel | [58][81] | 2021 |
| 15 | Graphene | Hydrothermal thermal dialysis and freeze-drying |
Excellent biocompatibility | [49][72] | 2021 |
| 16 | Chitin | Supercritical CO2 drying | Lower haemolysis ratio (<1%) | [70][93] | 2019 |
| 17 | Alginate | Supercritical CO2 drying | Not cytotoxic | [65][88] | 2020 |
| 18 | Cellulose | Supercritical CO2 drying | Excellent conditions for cell viability and proliferation | [71][94] | 2021 |
| 19 | Magnetic | Sol–gel | Biocompatible | [72][95] | 2022 |
| 20 | Silica | Sol–gel, supercritical drying |
Biocompatible for local and non- invasive drug delivery |
[60][83] | 2022 |
