Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Mark L Ormiston.
The natural killer (NK) cells of the immune system identify and remove stressed, infected, or cancerous cells in the body. This anti-tumor functionality has been harnessed through promising cell-based therapies that involve the isolation, expansion, activation, and delivery of NK cells for the treatment of several cancers.
- NK cell dysfunction
- NK cell plasticity
- TGFβ
1. Primary NK Cell Sources
To date, therapies that aim to harness the anti-tumor function of NK cells have involved either the adoptive transfer of ex vivo expanded NK cells, with or without genetic modification [15[1][2][3][4],16,17,18], or the promotion of in vivo NK cell activation through the administration of recombinant proteins, such as interleukin-15 (IL-15) superagonists [19,20][5][6]. For cell-based approaches, several sources have been explored, including primary NK cells from autologous or allogeneic peripheral blood, apheresis products, or umbilical cord blood (UCB) (Figure 1). Primary NK cells comprise only 10% of all lymphocytes in peripheral blood, limiting the number of cells that can be harvested for therapeutic applications and necessitating extensive purification methods, such as the magnetic depletion of undesired cell types [21][7]. NK cells are comparatively enriched in UCB, comprising 15–30% of total lymphocytes, but are immature and may be less cytotoxic compared to other sources [22,23][8][9].
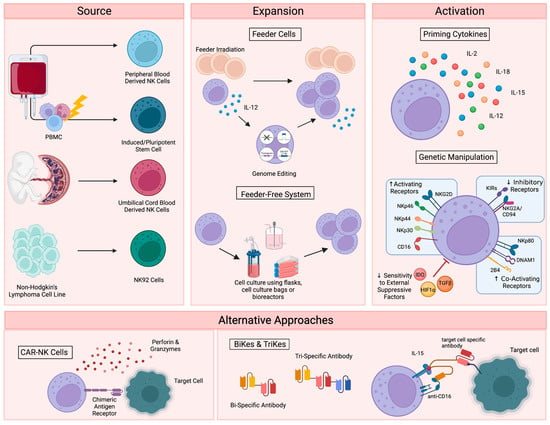
Figure 1. Current NK cell therapies. Schematic showing the current use of NK cell therapies, starting with source, expansion, and activation, then demonstrating the recently developed alternative approaches. Figure created with BioRender.
Alternatively, NK cells can be safely generated from CD34+ hematopoietic progenitors, induced pluripotent stem cells (iPSC), or embryonic stem cells [24,25][10][11]. While iPSC-derived NK cells offer a potentially infinite source of homogenously differentiated NK cells and can be functionally enhanced by genetic modification with relative ease, few groups have successfully created functional NK cells from this cellular source [26][12]. Woan and colleagues reported the use of a clonal iPSC-NK cell line, engineered to express both a high-affinity, non-cleavable version of the CD16a Fc receptor and a membrane-bound IL-15/IL-15 receptor-α fusion protein [27][13]. These cells demonstrated potent anti-tumor activity in vitro, as well as in myeloma and acute myeloid leukemia (AML) xenograft models. Clinical trials are currently underway [28][14]. The use of UCB-derived CD34+ cells as a source for NK cell therapies is also being explored by Deverra Therapeutics, through their non-engineered product DVX201 [29][15].
2. Immortalized NK Cell Lines
Immortalized NK cell lines also offer an alternative to primary NK cells, as they can be cultured indefinitely and are amenable to genetic manipulation. Several NK cell lines have been explored for therapeutic use, including NKL, NKG, NK-YS, YT, YTS, NK-92 cells, and high-affinity NK cells (haNKs). Originally isolated from an individual with non-Hodgkin’s lymphoma, NK-92 cells display high levels of anti-tumor cytotoxicity more consistently and reproducibly than other cell lines [30[16][17],31], are easily expandable to the clinical grade levels required for treatment [32][18], and have been used in recent clinical trials in patients with advanced, treatment-refractory malignancies [33,34][19][20]. HaNKs are NK-92 cells that have been engineered to express a high-affinity CD16 receptor for increased ADCC and endogenous production of IL-2 to maintain cytotoxic function [35,36][21][22]. While advantageous, the clinical application of these cells is not without limitation. NK-92 cells must be irradiated to protect from malignant expansion, potentially limiting their in vivo efficacy and persistence.
3. NK Cell Expansion and Activation
Regardless of their source, NK cells must be expanded to generate sufficient numbers of highly functional cells for therapeutic applications (Figure 1). Clinical scale expansion can be achieved using mitotically inactivated feeder cells, such as peripheral blood mononuclear cells (PBMCs), K562 or Jurkat cells, with or without genetic modification [37,38,39][23][24][25]. Relative to freshly isolated NK cells, expanded populations adopt an activated phenotype with increased expression of activating receptors NKG2D, NKG2C, NKp30, NKp44, DNAM-1 and key effector molecules, such as TRAIL, FasL and granzymes [40][26].
NK cells have also been successfully expanded in feeder-free systems using cell culture flasks, bags, or bioreactors in the presence of high doses of cytokines, such as IL-15 and IL-21 [41[27][28],42], cytokine-conjugated magnetic beads [43[29][30],44], or plasma membrane-derived particles [45][31]. However, these methods typically yield lower cell numbers than what is achieved with feeder cell-based expansion. More recently, advanced systems involving dissolvable polymer-based microspheres [46][32] or streamlined expansion protocols [47][33] have allowed for improved yields and the incorporation of nonviral genome editing into feeder-free expansion systems.
Although growing evidence supports the safety and efficacy of the majority of these expansion techniques [48][34], ex vivo NK cell expansion introduces the potential for senescence and exhaustion [49][35], resulting in the need for expanded NK cells to be activated or “primed” with cytokines like IL-2, IL-12, IL-15, and IL-18 to achieve maximal efficacy on tumors [50,51][36][37]. These cytokines, along with IL-21 and type I interferons, are central to the maturation, activation and survival of NK cells. IL-2 is critical to NK activation, can rejuvenate exhausted NK cells, and restores or preserves their cytotoxic potential in response to various stressors or following exposure to multiple myeloma [52,53,54][38][39][40]. Similarly, the stimulation of NK cells with various combinations of IL-2, IL-12, IL-15 and IL-18 increases the production of critical effector cytokines like IFNγ, IL-8, and TNF-α [55][41], enhances the responsiveness of the cells to the integrin, LFA-1, and improves subsequent receptor stimulation [56][42].
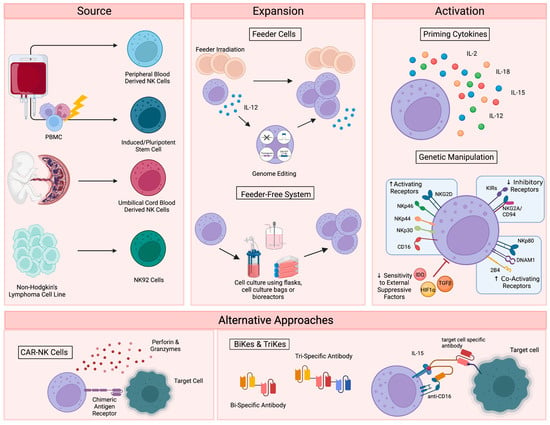
Figure 1. Current NK cell therapies. Schematic showing the current use of NK cell therapies, starting with source, expansion, and activation, then demonstrating the recently developed alternative approaches. Figure created with BioRender.
4. Alternative Approaches for NK Cell Activation
In addition to cytokine-based approaches, genetic manipulations, such as transgenic expression of the Chimeric Antigen Receptor (CAR), have also been explored as a means to enhance NK cell activation for therapeutic applications (Figure 1) [57][43]. CAR is a recombinant protein that can be introduced into cytotoxic lymphocytes to enable tumor antigen recognition and trigger activation [58][44]. CAR expression allows NK cells to specifically target cancer cells through recognition of tumor associated antigens. The generation of CAR-NK cells involves transducing isolated NK cells with CAR-encoding genes and expanding these cells prior to adoptive transfer into the patient [59][45]. Recently, UCB-derived CAR-NK cells were used to treat patients with relapsed or refractory CD19+ cancers, resulting in complete remission in the majority of patients without significant toxic effects [60][46]. Multiple clinical trials are currently underway [61][47], and ongoing studies are investigating the use of CAR-NK cells for the treatment of non-malignant targets, such as HIV-infected cells [62,63][48][49].
Despite these advantages, the complex CAR-NK cell manufacturing process is hindered by inefficient transduction methods [64][50], as well as the poor in vivo persistence shared by many NK cell therapy platforms. The personalized approach required for CAR-NK cells is also extremely expensive, time consuming, and difficult to apply large-scale. These limitations, which are not exclusive to CAR-NK approaches (Table 1), demonstrate a clear need for effective “off-the-shelf” therapies. One such approach is bi- and tri-specific antibodies (BiKEs and TriKEs), which trigger ADCC by binding both CD16 on NK cells and specific tumor antigens, creating a connection-like bridge between these cells and allowing for NK cell activation [65][51]. TriKEs have also been modified to incorporate cytokines like IL-15 to increase in vivo persistence and activation of NK cells [66][52]. In vitro, TriKEs have demonstrated enhanced NK cell activation and killing of both AML cell lines and patient-derived AML blasts [67][53]. A clinical trial in the treatment of high-risk hematological malignancies is currently underway [68][54].
Table 1. Advantages and Limitations to Current NK Cell Therapies.
| Advantages | Limitations | |
|---|---|---|
| Source | ||
| Peripheral Blood NK Cells | Reliable source of CD34 progenitor cells [64][50] | NK cells make up only ~10% of all lymphocytes in peripheral blood |
| High expression of CD16+ | Extensive purification is required to reduce contamination [21][7] | |
| Clinical studies have shown success with these cells after extensive enrichment and purification [69][55] | Isolating large amounts of PB NK cells is difficult [70,71,72][56][57][58] | |
| Cryopreservation has been shown to reduce cytotoxicity [71,73][57][59] | ||
| Umbilical Cord NK Cells | Greater abundance than PB NK cells (15–30% of total lymphocytes) [23][9] | UCB NK cells are immature |
| Fewer contaminating T cells in UCB than PB, reducing the risk of graft-versus-host disease [64][50] | ||
| Associated with good tolerance | May have reduced cytotoxic function [22][8] | |
| Minimal graft-vs-host-disease or toxicity [24][10] | ||
| Induced Pluripotent NK Cell | Easily genetically modified | Limited clinical success to date |
| High availability | Complex differentiation steps | |
| Ability to generate multiple doses from a single healthy donor [31,71][17][57] | Safety concerns regarding toxicity | |
| Commercial NK Cell Lines | Easy to obtain | Must undergo irradiation to prevent malignant expansion, which could limit persistence. |
| Highly cytotoxic | ||
| Easily expandable [32][18] | ||
| NK92 cells are the only cell line that has shown success in pre-clinical studies [31][17] | Efficiency of cells after expansion is variable (4–95%) [74][60] | |
| Expansion | ||
| Feeder Cells | Effective expansion of large numbers of NK cells [75][61]. | Difficult to maintain cytotoxic function after expansion [49][35]. |
| Feeder-Free Expansion | Large amounts of highly active NK cells have been produced | Cytotoxic function after expansion has not been well reported |
| Activation | ||
| IL-2 | Ability to restore NK cell cytotoxicity after exposure to various stressors [54][40]. | Systemic IL-2 leads to significant toxicity |
| Other Activating Cytokines | Less toxic than IL-2 | Thought to provide only minimal clinical benefit |
| Many combination therapies are required to provide a therapeutic benefit | ||
| Genetic Manipulation | Ability to target specific pathways of interest | Relatively newer area of study |
| Ability to avoid toxic effects associated with global therapies | ||
The NK cell engager platform (ANKET) has also been investigated as a means to harness NK cells as next-generation cancer immunotherapies. These NK cell engagers link a monoclonal antibody targeting the activating NK cell receptor NKp46 (or NKp30), an Fc fragment to promote ADCC via CD16, and an antibody targeting a tumor associated antigen, to enable the tumor-localized activation of host NK cells [76][62]. Work with these engagers has shown promise in targeting various cancer cell lines [76,77,78][62][63][64]. Recently, Demaria and colleagues reported a tetraspecific CD20-ANKET, which engages NKp46, CD16a, the beta chain of the IL-2 receptor, and a tumor associated antigen to induce preferential NK cell activation and target cell killing [79][65]. This tetraspecific CD20-ANKET induced the local control of tumors in non-human primates, without significant adverse side effects [79][65].
References
- Hercend, T.; Farace, F.; Baume, D.; Charpentier, F.; Droz, J.-P.; Triebel, F.; Escudier, B. Immunotherapy with Lymphokine-Activated Natural Killer Cells and Recombinant Interleukin-2: A Feasibility Trial in Metastatic Renal Cell Carcinoma. J. Biol. Response Mod. 1990, 9, 546–555.
- Miller, J.S.; Tessmer-Tuck, J.; Pierson, B.A.; Weisdorf, D.; McGlave, P.; Blazar, B.R.; Katsanis, E.; Verfaillie, C.; Lebkowski, J.; Radford, J., Jr. Low Dose Subcutaneous Interleukin-2 after Autologous Transplantation Generates Sustained In Vivo Natural Killer Cell Activity. Biol. Blood Marrow Transpl. 1997, 3, 34–44.
- Burns, L.J.; Weisdorf, D.J.; DeFor, T.E.; Vesole, D.H.; Repka, T.L.; Blazar, B.R.; Burger, S.R.; Panoskaltsis-Mortari, A.; Keever-Taylor, C.A.; Zhang, M.J. IL-2-Based Immunotherapy after Autologous Transplantation for Lymphoma and Breast Cancer Induces Immune Activation and Cytokine Release: A Phase I/II Trial. Bone Marrow Transpl. 2003, 32, 177–186.
- Liang, S.; Xu, K.; Niu, L.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Lin, M. Comparison of Autogeneic and Allogeneic Natural Killer Cells Immunotherapy on the Clinical Outcome of Recurrent Breast Cancer. Onco Targets Ther. 2017, 10, 4273.
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; Defor, T.E.; et al. First-in-Human Phase 1 Clinical Study of the IL-15 Superagonist Complex ALT-803 to Treat Relapse after Transplantation. Blood J. Am. Soc. Hematol. 2018, 131, 2515–2527.
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol. Res. 2016, 4, 49–60.
- Koehl, U.; Brehm, C.; Huenecke, S.; Kloess, S.; Bremm, M.; Zimmermann, S.Y.; Ullrich, E.; Soerensen, J.; Quaiser, A.; Erben, S.; et al. Clinical Grade Purification and Expansion of NK Cell Products for an Optimized Manufacturing Protocol. Front. Oncol. 2013, 3, 118.
- Harris, D.T.; Schumacher, M.J.; Locascio, J.; Besencon, F.J.; Olson, G.B.; DeLuca, D.; Shenker, L.; Bard, J.; Boyse, E.A. Phenotypic and Functional Immaturity of Human Umbilical Cord Blood T Lymphocytes. Proc. Natl. Acad. Sci. 1992, 89, 10006–10010.
- Luevano, M.; Daryouzeh, M.; Alnabhan, R.; Querol, S.; Khakoo, S.; Madrigal, A.; Saudemont, A. The Unique Profile of Cord Blood Natural Killer Cells Balances Incomplete Maturation and Effective Killing Function upon Activation. Hum. Immunol. 2012, 73, 248–257.
- Dolstra, H.; Roeven, M.W.H.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C. Successful Transfer of Umbilical Cord Blood CD34+ Hematopoietic Stem and Progenitor-Derived NK Cells in Older Acute Myeloid Leukemia PatientsHSPC-NK Cell Adoptive Transfer in Older AML Patients. Clin. Cancer Res. 2017, 23, 4107–4118.
- Spanholtz, J.; Tordoir, M.; Eissens, D.; Preijers, F.; van der Meer, A.; Joosten, I.; Schaap, N.; de Witte, T.M.; Dolstra, H. High Log-Scale Expansion of Functional Human Natural Killer Cells from Umbilical Cord Blood CD34-Positive Cells for Adoptive Cancer Immunotherapy. PLoS ONE 2010, 5, e9221.
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.N.; Lee, D.A.; Kaufman, D.S. Clinical-Scale Derivation of Natural Killer Cells from Human Pluripotent Stem Cells for Cancer Therapy. Stem Cells Transl. Med. 2013, 2, 274–283.
- Woan, K.V.; Kim, H.; Bjordahl, R.; Davis, Z.B.; Gaidarova, S.; Goulding, J.; Hancock, B.; Mahmood, S.; Abujarour, R.; Wang, H. Harnessing Features of Adaptive NK Cells to Generate IPSC-Derived NK Cells for Enhanced Immunotherapy. Cell Stem Cell 2021, 28, 2062–2075.
- FT538 in Subjects with Advanced Hematologic Malignancies—Full Text View ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04614636. (accessed on 10 March 2023).
- Safety and Efficacy of Allogeneic NK Cell Infusions in Patients with Relapsed/Refractory AML and High Risk MDS—Full Text View ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04901416 (accessed on 10 March 2023).
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a Human Cell Line (NK-92) with Phenotypical and Functional Characteristics of Activated Natural Killer Cells. Leukemia 1994, 8, 652–658.
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural Killer Cells for Immunotherapy—Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016, 7, 91.
- Tarn, Y.K.; Martinson, J.A.; Doligosa, K.; Klingernann, H.-G. Ex Vivo Expansion of the Highly Cytotoxic Human Natural Killer Cell Line NK-92 under Current Good Manufacturing Practice Conditions for Clinical Adoptive Cellular Immunotherapy. Cytotherapy 2003, 5, 259–272.
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.-Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 Clinical Trial of Adoptive Immunotherapy Using “off-the-Shelf” Activated Natural Killer Cells in Patients with Refractory and Relapsed Acute Myeloid Leukemia. Cytotherapy 2017, 19, 1225–1232.
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of Patients with Advanced Cancer with the Natural Killer Cell Line NK-92. Cytotherapy 2013, 15, 1563–1570.
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Maurice Morillon, Y.I.; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Yok Tsang, K.; Campbell, K.S.; et al. An NK Cell Line (HaNK) Expressing High Levels of Granzyme and Engineered to Express the High Affinity CD16 Allele. Oncotarget 2016, 7, 86359.
- Fabian, K.P.; Hodge, J.W. The Emerging Role of Off-the-Shelf Engineered Natural Killer Cells in Targeted Cancer Immunotherapy. Mol. Ther. Oncolytics 2021, 23, 266–276.
- Bae, D.S.; Lee, J.K. Development of NK Cell Expansion Methods Using Feeder Cells from Human Myelogenous Leukemia Cell Line. Blood Res. 2014, 49, 154–161.
- Kweon, S.; Phan, M.T.T.; Chun, S.; Yu, H.B.; Kim, J.; Kim, S.; Lee, J.; Ali, A.K.; Lee, S.H.; Kim, S.K.; et al. Expansion of Human NK Cells Using K562 Cells Expressing OX40 Ligand and Short Exposure to IL-21. Front. Immunol. 2019, 10, 879.
- Lim, S.A.; Kim, T.J.; Lee, J.E.; Sonn, C.H.; Kim, K.; Kim, J.; Choi, J.G.; Choi, I.K.; Yun, C.O.; Kim, J.H.; et al. Ex Vivo Expansion of Highly Cytotoxic Human NK Cells by Cocultivation with Irradiated Tumor Cells for Adoptive Immunotherapy. Cancer Res. 2013, 73, 2598–2607.
- Gurney, M.; Kundu, S.; Pandey, S.; O’Dwyer, M. Feeder Cells at the Interface of Natural Killer Cell Activation, Expansion and Gene Editing. Front. Immunol. 2022, 13, 802906.
- Sutlu, T.; Stellan, B.; Gilljam, M.; Quezada, H.C.; Nahi, H.; Gahrton, G.; Alici, E. Clinical-Grade, Large-Scale, Feeder-Free Expansion of Highly Active Human Natural Killer Cells for Adoptive Immunotherapy Using an Automated Bioreactor. Cytotherapy 2010, 12, 1044–1055.
- Wagner, J.; Pfannenstiel, V.; Waldmann, A.; Bergs, J.W.J.; Brill, B.; Huenecke, S.; Klingebiel, T.; Rödel, F.; Buchholz, C.J.; Wels, W.S. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front. Immunol. 2017, 8, 676.
- Li, X.; He, C.; Liu, C.; Ma, J.; Ma, P.; Cui, H.; Tao, H.; Gao, B. Expansion of NK Cells from PBMCs Using Immobilized 4-1BBL and Interleukin-21. Int. J. Oncol. 2015, 47, 335–342.
- Gras Navarro, A.; Kmiecik, J.; Leiss, L.; Zelkowski, M.; Engelsen, A.; Bruserud, Ø.; Zimmer, J.; Enger, P.Ø.; Chekenya, M. NK Cells with KIR2DS2 Immunogenotype Have a Functional Activation Advantage to Efficiently Kill Glioblastoma and Prolong Animal Survival. J. Immunol. 2014, 193, 6192–6206.
- Oyer, J.L.; Igarashi, R.Y.; Kulikowski, A.R.; Colosimo, D.A.; Solh, M.M.; Zakari, A.; Khaled, Y.A.; Altomare, D.A.; Copik, A.J. Generation of Highly Cytotoxic Natural Killer Cells for Treatment of Acute Myelogenous Leukemia Using a Feeder-Free, Particle-Based Approach. Biol. Blood Marrow Transplant. 2015, 21, 632–639.
- Johnson, C.D.L.; Zale, N.E.; Frary, E.D.; Lomakin, J.A. Feeder-Cell-Free and Serum-Free Expansion of Natural Killer Cells Using Cloudz Microspheres, G-Rex6M, and Human Platelet Lysate. Front. Immunol. 2022, 13, 803380.
- Huang, R.-S.; Lai, M.-C.; Shih, H.-A.; Lin, S. A Robust Platform for Expansion and Genome Editing of Primary Human Natural Killer Cells. J. Exp. Med. 2021, 218, e20201529.
- Felices, M.; Lenvik, A.J.; McElmurry, R.; Chu, S.; Hinderlie, P.; Bendzick, L.; Geller, M.A.; Tolar, J.; Blazar, B.R.; Miller, J.S. Continuous Treatment with IL-15 Exhausts Human NK Cells via a Metabolic Defect. JCI. Insight. 2018, 3, e96219.
- Suen, W.C.-W.; Lee, W.Y.-W.; Leung, K.-T.; Pan, X.-H.; Li, G. Natural Killer Cell-Based Cancer Immunotherapy: A Review on 10 Years Completed Clinical Trials. Cancer Investig. 2018, 36, 431–457.
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057.
- Ni, J.; Miller, M.; Stojanovic, A.; Garbi, N.; Cerwenka, A. Sustained Effector Function of IL-12/15/18–Preactivated NK Cells against Established Tumors. J. Exp. Med. 2012, 209, 2351–2365.
- Lehmann, C.; Zeis, M.; Uharek, L. Activation of Natural Killer Cells with Interleukin 2 (IL-2) and IL-12 Increases Perforin Binding and Subsequent Lysis of Tumour Cells. Br. J. Haematol. 2001, 114, 660–665.
- Bhat, R.; Watzl, C. Serial Killing of Tumor Cells by Human Natural Killer Cells—Enhancement by Therapeutic Antibodies. PLoS ONE 2007, 2, e326.
- Sarkar, S.; Germeraad, W.T.V.; Rouschop, K.M.A.; Steeghs, E.M.P.; van Gelder, M.; Bos, G.M.J.; Wieten, L. Hypoxia Induced Impairment of NK Cell Cytotoxicity against Multiple Myeloma Can Be Overcome by IL-2 Activation of the NK Cells. PLoS ONE 2013, 8, e64835.
- Poznanski, S.M.; Lee, A.J.; Nham, T.; Lusty, E.; Larché, M.J.; Lee, D.A.; Ashkar, A.A. Combined Stimulation with Interleukin-18 and Interleukin-12 Potently Induces Interleukin-8 Production by Natural Killer Cells. J. Innate Immun. 2017, 9, 511–525.
- Urlaub, D.; Höfer, K.; Müller, M.-L.; Watzl, C. LFA-1 Activation in NK Cells and Their Subsets: Influence of Receptors, Maturation, and Cytokine Stimulation. J. Immunol. 2017, 198, 1944–1951.
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine 2020, 59, 102975.
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398.
- Khawar, M.B.; Sun, H. CAR-NK Cells: From Natural Basis to Design for Kill. Front. Immunol. 2021, 12, 5157.
- Liu, S.; Galat, V.; Galat4, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 7.
- Albinger, N.; Hartmann, J.; Ullrich, E. Current Status and Perspective of CAR-T and CAR-NK Cell Therapy Trials in Germany. Gene Ther. 2021, 28, 513–527.
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553.
- Daher, M.; Melo Garcia, L.; Li, Y.; Rezvani, K. CAR-NK Cells: The next Wave of Cellular Therapy for Cancer. Clin. Transl. Immunology. 2021, 10, e1274.
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric Antigen Receptor-Engineered Natural Killer Cells for Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 168.
- Felices, M.; Lenvik, T.R.; Davis, Z.B.; Miller, J.S.; Vallera, D.A. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods. Mol. Biol. 2016, 1441, 333–346.
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, in Vivo Expansion, and En-hanced Function. Clin. Cancer. Res. 2016, 22, 3440–3450.
- Arvindam, U.S.; van Hauten, P.M.M.; Schirm, D.; Schaap, N.; Hobo, W.; Blazar, B.R.; Vallera, D.A.; Dolstra, H.; Felices, M.; Miller, J.S. A Trispecific Killer Engager Molecule against CLEC12A Effectively Induces NK-Cell Mediated Killing of AML Cells. Leukemia 2021, 35, 1586–1596.
- GTB-3550 Tri-Specific Killer Engager (TriKE ®) for High Risk Hematologic Malignancies—Full Text View Clini-calTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03214666 (accessed on 10 March 2023).
- Williams, S.M.; Sumstad, D.; Kadidlo, D.; Curtsinger, J.; Luo, X.; Miller, J.S.; McKenna Jr, D.H. Clinical-scale Production of CGMP Compliant CD3/CD19 Cell-depleted NK Cells in the Evolution of NK Cell Immunotherapy at a Single Institution. Transfusion 2018, 58, 1458–1467.
- Passweg, J.R.; Tichelli, A.; Meyer-Monard, S.; Heim, D.; Stern, M.; Kühne, T.; Favre, G.; Gratwohl, A. Purified Donor NK-Lymphocyte Infusion to Consolidate Engraftment after Haploidentical Stem Cell Transplantation. Leukemia 2004, 18, 1835–1838.
- Shankar, K.; Capitini, C.M.; Saha, K. Genome Engineering of Induced Pluripotent Stem Cells to Manufacture Natural Killer Cell Therapies. Stem Cell Res. Ther. 2020, 11, 234.
- Yoon, S.R.; Lee, Y.S.; Yang, S.H.; Ahn, K.H.; Lee, J.-H.; Lee, J.-H.; Kim, D.Y.; Kang, Y.A.; Jeon, M.; Seol, M. Generation of Donor Natural Killer Cells from CD34+ Progenitor Cells and Subsequent Infusion after HLA-Mismatched Allogeneic Hematopoietic Cell Transplantation: A Feasibility Study. Bone Marrow Transpl. 2010, 45, 1038–1046.
- Mark, C.; Czerwinski, T.; Roessner, S.; Mainka, A.; Hörsch, F.; Heublein, L.; Winterl, A.; Sanokowski, S.; Richter, S.; Bauer, N. Cryopreservation Impairs 3-D Migration and Cytotoxicity of Natural Killer Cells. Nat. Commun. 2020, 11, 5224.
- Matosevic, S. Viral and Nonviral Engineering of Natural Killer Cells as Emerging Adoptive Cancer Immunotherapies. J. Immunol. Res. 2018, 2018, 4054815.
- Somanchi, S.S.; Somanchi, A.; Cooper, L.J.N.; Lee, D.A. Engineering Lymph Node Homing of Ex Vivo–Expanded Human Natural Killer Cells via Trogocytosis of the Chemokine Receptor CCR7. Blood J. Am. Soc. Hematol. 2012, 119, 5164–5172.
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16.
- Colomar-Carando, N.; Gauthier, L.; Merli, P.; Loiacono, F.; Canevali, P.; Falco, M.; Galaverna, F.; Rossi, B.; Bosco, F.; Caratini, M.; et al. Exploiting Natural Killer Cell Engagers to Control Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. Cancer Immunol. Res. 2022, 10, 291–302.
- Gauthier, L.; Virone-Oddos, A.; Beninga, J.; Rossi, B.; Nicolazzi, C.; Amara, C.; Blanchard-Alvarez, A.; Gourdin, N.; Courta, J.; Basset, A.; et al. Control of Acute Myeloid Leukemia by a Trifunctional NKp46-CD16a-NK Cell Engager Targeting CD123. Nat. Biotechnol. 2023.
- Demaria, O.; Gauthier, L.; Vetizou, M.; Blanchard Alvarez, A.; Vagne, C.; Habif, G.; Batista, L.; Baron, W.; Belaïd, N.; Girard-Madoux, M.; et al. Antitumor Immunity Induced by Antibody-Based Natural Killer Cell Engager Therapeutics Armed with Not-Alpha IL-2 Variant. Cell Rep. Med. 2022, 3, 100783.
More
