You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Frans B Plotz.
The difficulty in recognizing early-onset neonatal sepsis (EONS) in a timely manner due to non-specific symptoms and the limitations of diagnostic tests, combined with the risk of serious consequences if EONS is not treated in a timely manner, has resulted in a low threshold for starting empirical antibiotic treatment. New guideline strategies, such as the neonatal sepsis calculator, have been proven to reduce the antibiotic burden related to EONS, but lack sensitivity for detecting EONS.
- antibiotics
- biomarkers
- blood culture
- early-onset neonatal sepsis
1. Introduction
Early-onset neonatal sepsis (EONS) is a rare but potentially life-threatening disease. It is defined as sepsis within 72 h after birth, confirmed with a positive blood and/or cerebrospinal fluid culture [1,2][1][2]. In Europe, the incidence of culture-proven EONS is estimated to be around 0.5–1 per 1000 live newborns [3]. The predominant pathogen is Group B Streptococcus (GBS; Streptococcus agalactiae), which causes one-third to half of all EONS cases, followed by Escherichia coli (E. coli) [4,5][4][5].
The difficulty in recognizing EONS in a timely manner due to non-specific symptoms and limitations in the time-effectiveness and accuracy of diagnostic testing, combined with the risk of serious consequences if EONS is not treated in a timely manner, has resulted in a low threshold for starting empirical antibiotic treatment. This has led to significant overtreatment, despite the well-known short- and long-term side effects [6,7,8,9,10,11,12][6][7][8][9][10][11][12]. In addition, despite a negative blood culture, antibiotic therapy is continued for more than three days in about 30% of newborns once started [13,14][13][14]. Suspicion of the quality of the blood culture, for example based on incorrectly obtained cultures, low sensitivity and the use of intrapartum antibiotic prophylaxis, are reported reasons for continuing antibiotic therapy [15]. Consequently, culture-negative sepsis is frequently diagnosed, although the actual incidence or even the existence of such a phenomenon is uncertain, as plenty of studies have used prolonged antibiotic treatment as a diagnostic criterion.
2. Maternal Perspective
2.1. GBS Prevention
Most countries use a screening strategy that recommends screening pregnant women between 35 and 37 weeks of gestation for GBS carriage [22][16]. Those who are GBS carriers receive intrapartum antibiotic prophylaxis (IAP) [23][17]. Countries that have introduced such a screening strategy have observed a substantial reduction in EONS. In the case of risk-based screening, only pregnant women with certain risk factors are eligible for either screening or receiving IAP directly [24,25][18][19]. However, unexpectedly, these countries have not observed a decline in early-onset GBS disease [20,21][20][21]. The limited sensitivity of risk-based guidelines may explain their lack of effectiveness. Firstly, up to one-fifth of pregnant GBS carriers have no risk factors that would lead to IAP recommendations [26][22]. Secondly, culture results may be falsely negative, especially when the culture guidelines for the detection and identification of GBS are not followed [27,28][23][24]. These guidelines recommend sampling both the lower vagina and the rectum using a flocked swab and using adequate transport and enriched culture media in order to optimize sensitivity [27][23]. Thirdly, it usually takes 24 to 48 h for culture results to become available, which can lead to inadequate prophylaxis. Fourthly, there is low compliance to GBS risk-based guidelines [29][25]. False-negative culture results can also occur using the screening strategy. GBS carriage can be intermittent.2.2. Host–Pathogen Interactions in GBS Disease
The specificity of both screening- and risk-based IAP guidelines is limited, leading to high rates of unnecessary antibiotic treatment in women. Whether GBS transmission leads to harmless colonization or to invasive disease depends on the invasive potential of the GBS bacterium and the susceptibility of the hosts, i.e., the mother and her child. GBS express virulence factors to invade tissue barriers, evade host defense mechanisms and cause damage to host tissues in order to cause disease. Examples of virulence factors are α-like proteins that enable cell invasion, the polysaccharide capsule that causes host immune evasion and the hyper-virulent GBS surface-anchored adhesin protein (HvgA) that enables crossing of the blood–brain barrier [33,34][26][27]. These virulence factors are coded in bacterial virulence genes, and these genes vary among GBS subtypes. Epidemiological research has consistently shown that specific GBS bacterial genotypes, such as CC17, are over-represented in invasive disease compared with colonization, strongly suggesting that invasive GBS strains have unique bacterial virulence genes [20,35,36,37,38,39,40][20][28][29][30][31][32][33]. The specificity of IAP strategies could possibly be improved not only by screening for GBS carriage, but also by incorporating multiple genetic markers into a single PCR test, making it possible to determine both the presence and the invasive potential of GBS bacteria [37][30].2.3. Maternal Immunization
Another promising strategy to prevent invasive GBS disease is maternal immunization during pregnancy. Lower antibody titers against the GBS polysaccharide capsule and some GBS surface proteins in uncolonized pregnant women are associated with a higher probability of becoming colonized during pregnancy [45,46][34][35]. Vaccination may prevent the transmission of GBS from mother to child by reducing GBS carriage [47][36]. More importantly, the transfer of protective IgG antibodies to the newborn via the placenta might protect the child from invasive GBS disease during the first months of life. A hexavalent GBS conjugate vaccine was recently proven safe and immunogenic in healthy adults and is currently being studied in pregnant women [48][37]. However, clinical efficacy studies are time-consuming and expensive due to low disease incidence. Since antibody levels are related to the risk of invasive disease, determining the serological immune correlates of protection is a promising approach that may accelerate the licensure of a maternal GBS vaccine [49][38].2.4. Antepartum Immunological Biomarkers
Chorioamnionitis is a heterogeneous condition characterized by intrauterine infection, inflammation or both and is a common cause of preterm birth and adverse neonatal outcomes. Nevertheless, the actual intrauterine transmission of micro-organisms to the newborn that result in EONS is limited, and the risk of EONS in chorioamnionitis cases in well-appearing newborns after birth is <1% [50,51][39][40]. In an attempt to differentiate between infectious and non-infectious causes of maternal fever and causes of amniotic fluid infection that could result in EONS, experts introduced the term ‘triple I’ to aid clinical decision making. Triple I (infection, inflammation or both) is based on clinical characteristics, maternal white blood cell counts, bacterial cultures and the histological evidence of placental infection and/or fetal membranes [52][41]. Maternal immunological biomarkers that differentiate between inflammation and infection are potentially interesting diagnostic targets to identify those at risk. In addition, the material is relatively easy to obtain, and by investigating the mother’s condition, there is the potential to start appropriate treatment early in the course of the disease. These biomarkers are of particular interest in women with preterm labor who are being evaluated for tocolysis.3. Umbilical Cord Perspective
3.1. Biomarkers
The use of umbilical cord blood biomarkers to diagnose newborns with EONS could provide information early in the course of the disease, even before clinical signs are apparent. Postnatal umbilical cord sampling is non-invasive, painless and easy to perform, and a larger volume of blood is available for testing than in the case of sampling postpartum in the newborn. This is particularly important for neonates born with an extremely low birth weight. A large range of biomarkers have been tested in the umbilical cord blood of preterm and term newborns, for example, CRP, procalcitonin (PCT), interleukins, TNF-α, interferon gamma (IFN-y), serum amyloid A (SAA), presepsin, etc. [48,49,50,51,52,53][37][38][39][40][41][42]. Similar to maternal serum samples, CRP does not perform well in distinguishing between infected and non-infected newborns, with low sensitivity (around 50%) across studies [53][42]. The specificity and negative predictive value of CRP are slightly better, meaning that low CRP levels might be helpful in identifying newborns who are at low risk of EONS. More interesting biomarkers include PCT and IL-6, which have performed better than CRP and most of the tested interleukins in the majority of studies [55,56][43][44]. Recent meta-analyses have shown a pooled sensitivity >80% and a pooled specificity >92% for PCT (in eight studies), and a pooled sensitivity of 76–78% and a pooled specificity of 79–82% for IL-6 [53,57,58][42][45][46]. The combination of PCT and IL-6 is less consistent but still promising, with sensitivity ranging between 46% and 91% and specificity between 77% and 99%.3.2. Blood Culture and Molecular Techniques
Although conventional peripheral bacterial cultures are considered the gold standard in order to establish a diagnosis of EONS, its sensitivity has been questioned. The majority of infants treated with antibiotics for presumed EONS have negative blood cultures and have so-called culture-negative EONS [62][47]. Multiple factors may influence the risk of false-negative blood cultures, and thus impact sensitivity. Low bacterial loads in infants with EONS, inadequate blood volume collection and exposure to maternal intrapartum antibiotics may increase the risk of false-negative blood cultures, which can decrease sensitivity and challenge the reliability of peripheral bacterial culture as the gold standard [63][48]. The American Academy of Pediatrics guidelines for the evaluation of early- and late-onset sepsis recommend obtaining a minimum blood sample of 1 mL, as there is a direct correlation between the volume of blood obtained and the sensitivity of the blood culture [64][49].4. Newborn Perspective
4.1. Hematological Cell Indices
Hematological cell indices have been used for decades in order to aid the diagnosis of EONS, but their diagnostic performance as individual markers is poor. In general, indices, such as complete blood cell counts, show low sensitivity and relatively high specificity, meaning that sepsis could not be ruled out in cases of normal cell counts [53,70][42][50]. Platelets have recently gained renewed interest as first-line responders to infection and as biomarkers for EONS. Thrombocytopenia is seen in almost half of the NICU patients with early- or late-onset sepsis. Moreover, an increased mean platelet volume (MPV) has been observed in patients with EONS compared with healthy controls [71,72][51][52].4.2. Biomarkers
The perfect biomarker should be able to differentiate between newborns with an infection and those with symptoms associated with the transition after birth that mimics EONS. Additionally, a biomarker should identify a well-appearing newborn without maternal risk factors that will develop EONS hours after birth, and the results should be available as early as possible. Testing for host markers that change in response to infection would require a smaller blood volume and would result in quicker results than culture-based methods. Various systematic reviews show that a single biomarker with optimal diagnostic accuracy does not exist to date, but numerous researchers have attempted to find one [73,74][53][54]. The enormous number of studies focusing on a single biomarker to aid in the diagnosis of EONS has led to the realization that one biomarker is not sufficient. Even though numerous promising markers exist, their evaluation and validation in large cohorts are often missing. Meta-analyses are hampered by the large heterogeneity in case definitions, cut-off values and the techniques used. Among the most commonly used and studied biomarkers are acute-phase proteins CRP, PCT and IL-6. CRP performs poorly as an individual marker in diagnosing EONS early in the course of the disease. PCT is described to be less reliable after antepartum antibiotics are administered, and widely ranging sensitivity and specificity have been described [53,77,78][42][55][56]. IL-6 shows diagnostic accuracy, with a reported pooled sensitivity of 72% and a pooled specificity of 75%, which makes it not satisfactory enough to use as a single diagnostic marker [53][42].4.3. Blood Culture and Molecular Techniques
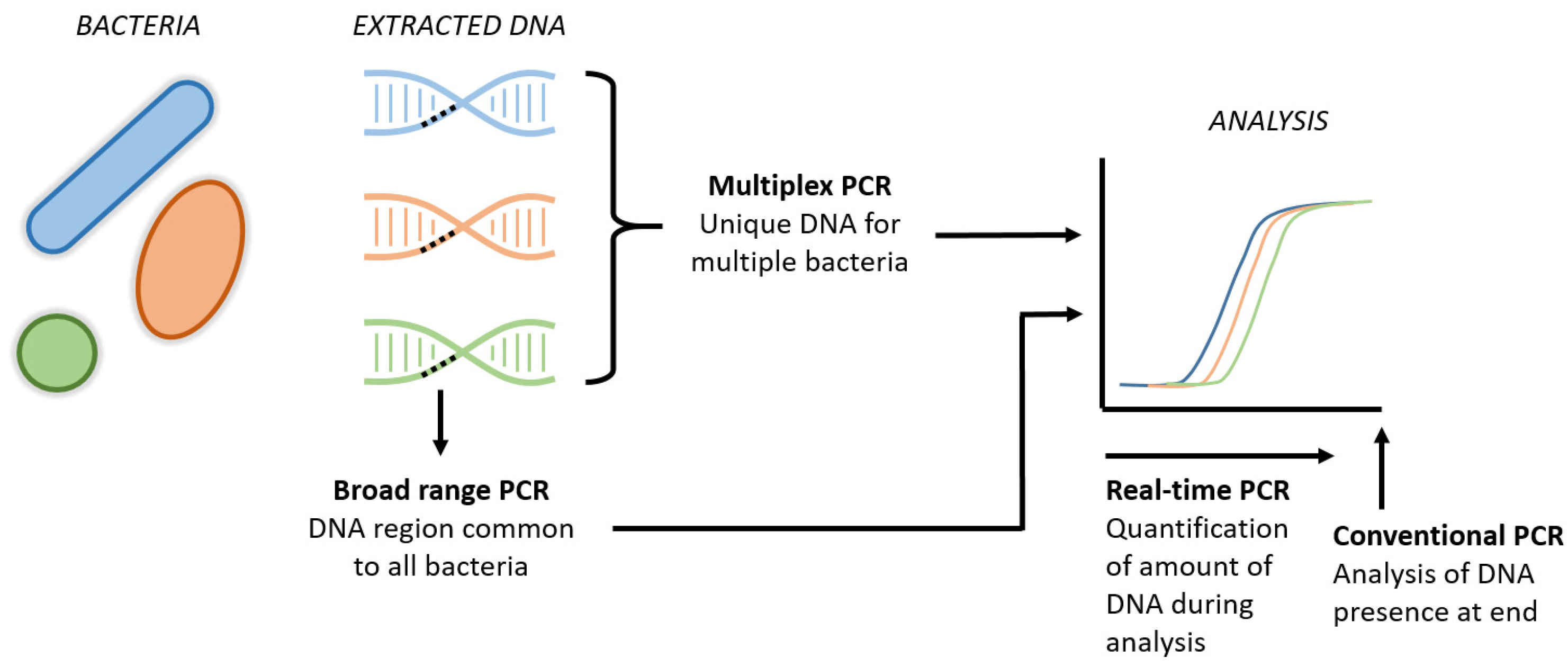
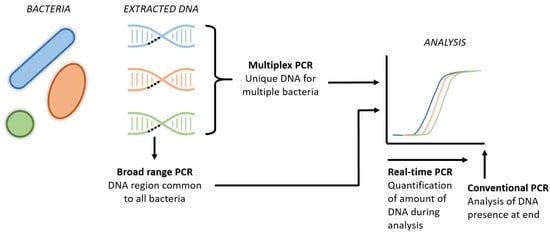
The two major drawbacks of conventional peripheral blood cultures include a delay in the availability of results of up to 48–72 h following sampling and the questioned sensitivity, as discussed above. Inertia prevents the traditional blood culture from serving as a diagnostic test to exclude EONS directly postpartum [82][57]. Rapid diagnostic tools with high sensitivity and a negative predictive value within hours after birth could result in a significant reduction in the overuse of antibiotics in uninfected newborns. Molecular techniques, such as broad-range PCR, real-time PCR and multiplex PCR, generate results faster than conventional blood cultures (Figure 21).

Figure 21. Schematic visualization of new PCR techniques. The broad range PCRs refers to the analysis of a common region present in all bacteria, aiming to detect the presence of bacterial DNA in general. Multiplex PCR includes the simultaneous DNA analysis of several specific bacteria in one tube. Real-time PCR is the new technique that enables us to quantify the amount of DNA throughout the entire analysis, instead of the presence of DNA at the end of the analysis in conventional PCR.
However, these PCR techniques are restricted by the panel used and can only detect a predefined set of pathogens. Unrestricted techniques, such as 16S sequencing, on the other hand, are costly and have a reporting delay of one to several days, which is comparable to conventional culturing. A novel technique called Molecular Culture using IS-pro (inBiome, Amsterdam, the Netherlands) is an unrestricted PCR-based technique that allows the profiling of all the bacterial species present in a sample to be conducted within 5 h, but its potential has not yet been investigated in EONS [86][58].
4.4. Clinical Prediction and Monitoring
Major advancements towards the better use of antibiotics postpartum have been realized with the development of the neonatal sepsis calculator by the research division of Kaiser Permanente in the United States [88,89][59][60]. Based on a large dataset, objective clinical maternal and neonatal parameters are used in an accessible clinical risk calculator that provides guidance on the use of antibiotics after birth. This method is associated with a safe and effective reduction in the rate of antibiotic treatment after birth [17][61]. However, post-development analyses and data evaluating this model suggest an untapped potential for the improvement of this type of clinical monitoring and related risk stratification; the overtreatment of non-infected newborns with antibiotics remains significant, whereas the sepsis calculator does not identify EONS cases better than the alternative methods [18,19,90][62][63][64].5. Conclusions
In order to further decrease the number of newborns who are empirically treated with antibiotic therapy and to reduce the duration of antibiotic therapy in the case of a negative blood culture, it is necessary to develop novel strategies aimed at preventing EONS and at increasing diagnostic sensitivity for detecting EONS. Promising strategies from the maternal perspective include GBS prevention, exploring the virulence factors of GBS, maternal immunization and antepartum biomarkers. The diagnostic methods obtained from the umbilical cord are preliminary and, although promising, in general not yet ready for implementation in daily practice. The most promising and extensively studied fields from the newborn perspective are biomarkers, new microbiological techniques and clinical prediction and monitoring strategies.References
- Seale, A.C.; Bianchi-Jassir, F.; Russell, N.J.; Kohli-Lynch, M.; Tann, C.J.; Hall, J.; Madrid, L.; Blencowe, H.; Cousens, S.; Baker, C.J.; et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin. Infect. Dis. 2017, 65, S200–S219.
- Vergnano, S.; Menson, E.; Smith, Z.; Kennea, N.; Embleton, N.; Clarke, P.; Watts, T.; Heath, P.T. Characteristics of Invasive Staphylococcus aureus in United Kingdom Neonatal Units. Pediatr. Infect. Dis. J. 2011, 30, 850–854.
- Giannoni, E.; Dimopoulou, V.; Klingenberg, C.; Naver, L.; Nordberg, V.; Berardi, A.; El Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; et al. Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia. JAMA Netw. Open 2022, 5, e2243691.
- Schrag, S.J.; Farley, M.M.; Petit, S.; Reingold, A.; Weston, E.J.; Pondo, T.; Hudson Jain, J.; Lynfield, R. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics 2016, 138, e20162013.
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D. Early-onset neonatal sepsis. Clin. Microbiol. Rev. 2014, 27, 21–47.
- Giannoni, E.; Agyeman, P.K.; Stocker, M.; Posfay-Barbe, K.M.; Heininger, U.; Spycher, B.D.; Bernhard-Stirnemann, S.; Niederer-Loher, A.; Kahlert, C.R.; Donas, A.; et al. Neonatal Sepsis of Early Onset, and Hospital-Acquired and Community-Acquired Late Onset: A Prospective Population-Based Cohort Study. J. Pediatr. 2018, 201, 106–114.e4.
- Walker, S.M. Long-term effects of neonatal pain. Semin. Fetal. Neonatal. Med. 2019, 24, 101005.
- Reyman, M.; van Houten, M.A.; Watson, R.L.; Chu, M.; Arp, K.; de Waal, W.J.; Schiering, I.; Plotz, F.B.; Willems, R.J.L.; van Schaik, W.; et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: A randomized trial. Nat. Commun. 2022, 13, 893.
- Scott, F.I.; Horton, D.B.; Mamtani, R.; Haynes, K.; Goldberg, D.S.; Lee, D.Y.; Lewis, J.D. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology 2016, 151, 120–129.e5.
- Droste, J.H.; Wieringa, M.H.; Weyler, J.J.; Nelen, V.J.; Vermeire, P.A.; Van Bever, H.P. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin. Exp. Allergy 2000, 30, 1547–1553.
- Zargari, I.; Adar, A.; Morag, I.; Pinhas-Hamiel, O.; Eyal, O.; Keidar, R.; Loewenthal, N.; Levy, M.; Dally-Gottfried, O.; Landau, Z.; et al. Early exposures and inherent factors in premature newborns are associated with type 1 diabetes. Pediatr. Res. 2022.
- Urban-Chmiel, R.; Marek, A.; Stepien-Pysniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria-A Review. Antibiotics 2022, 11, 1079.
- Mundal, H.S.; Ronnestad, A.; Klingenberg, C.; Stensvold, H.J.; Stordal, K. Antibiotic Use in Term and Near-Term Newborns. Pediatrics 2021, 148, e2021051339.
- van der Weijden, B.M.; Achten, N.B.; Bekhof, J.; Evers, E.E.; Berk, M.; Kamps, A.W.A.; Rijpert, M.; Ten Tusscher, G.W.; van Houten, M.A.; Plotz, F.B. Multicentre study found that adherence to national antibiotic recommendations for neonatal early-onset sepsis was low. Acta Paediatr. 2021, 110, 791–798.
- Cantey, J.B.; Baird, S.D. Ending the Culture of Culture-Negative Sepsis in the Neonatal ICU. Pediatrics 2017, 140, e20170044.
- Le Doare, K.; O’Driscoll, M.; Turner, K.; Seedat, F.; Russell, N.J.; Seale, A.C.; Heath, P.T.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; et al. Intrapartum Antibiotic Chemoprophylaxis Policies for the Prevention of Group B Streptococcal Disease Worldwide: Systematic Review. Clin. Infect. Dis. 2017, 65, S143–S151.
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion Summary, Number 782. Obstet. Gynecol. 2019, 134, e19–e40.
- The Dutch Society of Obstetrics and Gynaecology; The Dutch Pediatrics Association. Prevention and Treatment of Early-Onset Neonatal Infection (Adapted from NICE Guidelines); The Dutch Society of Obstetrics and Gynaecology: Utrecht, The Netherlands, 2017; pp. 1–97.
- Bedford Russell, A.R.; Kumar, R. Early onset neonatal sepsis: Diagnostic dilemmas and practical management. Arch. Dis. Child Fetal. Neonatal. Ed. 2015, 100, F350–F354.
- van Kassel, M.N.; de Boer, G.; Teeri, S.A.F.; Jamrozy, D.; Bentley, S.D.; Brouwer, M.C.; van der Ende, A.; van de Beek, D.; Bijlsma, M.W. Molecular epidemiology and mortality of group B streptococcal meningitis and infant sepsis in the Netherlands: A 30-year nationwide surveillance study. Lancet Microbe 2021, 2, e32–e40.
- Lamagni, T.L.; Keshishian, C.; Efstratiou, A.; Guy, R.; Henderson, K.L.; Broughton, K.; Sheridan, E. Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991–2010. Clin. Infect. Dis. 2013, 57, 682–688.
- Daniels, J.P.; Gray, J.; Pattison, H.M.; Gray, R.; Hills, R.K.; Khan, K.S.; Group, G.B.S.C. Intrapartum tests for group B streptococcus: Accuracy and acceptability of screening. BJOG 2011, 118, 257–265.
- American Society for Microbiology. Guidelines for the Detection and Identification of Group B Streptococcus; American Society for Microbiology: Washington, NW, USA, 2020.
- Prevention of Early-onset Neonatal Group B Streptococcal Disease: Green-top Guideline No. 36. BJOG 2017, 124, e280–e305.
- Kolkman, D.G.E.; Rijnders, M.E.B.; Wouters, M.; Dommelen, P.V.; de Groot, C.J.M.; Fleuren, M.A.H. Adherence to three different strategies to prevent early onset GBS infection in newborns. Women Birth 2020, 33, e527–e534.
- Tazi, A.; Bellais, S.; Tardieux, I.; Dramsi, S.; Trieu-Cuot, P.; Poyart, C. Group B Streptococcus surface proteins as major determinants for meningeal tropism. Curr. Opin. Microbiol. 2012, 15, 44–49.
- Zurn, K.; Lander, F.; Hufnagel, M.; Monecke, S.; Berner, R. Microarray Analysis of Group B Streptococci Causing Invasive Neonatal Early- and Late-onset Infection. Pediatr. Infect. Dis. J. 2020, 39, 449–453.
- Fluegge, K.; Wons, J.; Spellerberg, B.; Swoboda, S.; Siedler, A.; Hufnagel, M.; Berner, R. Genetic differences between invasive and noninvasive neonatal group B streptococcal isolates. Pediatr. Infect. Dis. J. 2011, 30, 1027–1031.
- Chen, S.L. Genomic Insights Into the Distribution and Evolution of Group B Streptococcus. Front. Microbiol. 2019, 10, 1447.
- Van Elzakker, E.; Yahiaoui, R.; Visser, C.; Oostvogel, P.; Muller, A.; Ho, Y.R.; Wu, J.J.; van Belkum, A. Epidemiology of and prenatal molecular distinction between invasive and colonizing group B streptococci in The Netherlands and Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 921–928.
- Almeida, A.; Rosinski-Chupin, I.; Plainvert, C.; Douarre, P.E.; Borrego, M.J.; Poyart, C.; Glaser, P. Parallel Evolution of Group B Streptococcus Hypervirulent Clonal Complex 17 Unveils New Pathoadaptive Mutations. mSystems 2017, 2, e00074-17.
- Jamrozy, D.; Bijlsma, M.W.; de Goffau, M.C.; van de Beek, D.; Kuijpers, T.W.; Parkhill, J.; van der Ende, A.; Bentley, S.D. Increasing incidence of group B streptococcus neonatal infections in the Netherlands is associated with clonal expansion of CC17 and CC23. Sci. Rep. 2020, 10, 9539.
- Chaguza, C.; Jamrozy, D.; Bijlsma, M.W.; Kuijpers, T.W.; van de Beek, D.; van der Ende, A.; Bentley, S.D. Population genomics of Group B Streptococcus reveals the genetics of neonatal disease onset and meningeal invasion. Nat. Commun. 2022, 13, 4215.
- Dzanibe, S.; Kwatra, G.; Adrian, P.V.; Kimaro-Mlacha, S.Z.; Cutland, C.L.; Madhi, S.A. Association between antibodies against group B Streptococcus surface proteins and recto-vaginal colonisation during pregnancy. Sci. Rep. 2017, 7, 16454.
- Kwatra, G.; Adrian, P.V.; Shiri, T.; Buchmann, E.J.; Cutland, C.L.; Madhi, S.A. Serotype-specific acquisition and loss of group B streptococcus recto-vaginal colonization in late pregnancy. PLoS ONE 2014, 9, e98778.
- Hillier, S.L.; Ferrieri, P.; Edwards, M.S.; Ewell, M.; Ferris, D.; Fine, P.; Carey, V.; Meyn, L.; Hoagland, D.; Kasper, D.L.; et al. A Phase 2, Randomized, Control Trial of Group B Streptococcus (GBS) Type III Capsular Polysaccharide-tetanus Toxoid (GBS III-TT) Vaccine to Prevent Vaginal Colonization With GBS III. Clin. Infect. Dis. 2019, 68, 2079–2086.
- Absalon, J.; Simon, R.; Radley, D.; Giardina, P.C.; Koury, K.; Jansen, K.U.; Anderson, A.S. Advances towards licensure of a maternal vaccine for the prevention of invasive group B streptococcus disease in infants: A discussion of different approaches. Hum. Vaccin. Immunother. 2022, 18, 2037350.
- Gilbert, P.B.; Isbrucker, R.; Andrews, N.; Goldblatt, D.; Heath, P.T.; Izu, A.; Madhi, S.A.; Moulton, L.; Schrag, S.J.; Shang, N.; et al. Methodology for a correlate of protection for group B Streptococcus: Report from the Bill & Melinda Gates Foundation workshop held on 10 and 11 February 2021. Vaccine 2022, 40, 4283–4291.
- Shakib, J.; Buchi, K.; Smith, E.; Young, P.C. Management of newborns born to mothers with chorioamnionitis: Is it time for a kinder, gentler approach? Acad. Pediatr. 2015, 15, 340–344.
- Kiser, C.; Nawab, U.; McKenna, K.; Aghai, Z.H. Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics 2014, 133, 992–998.
- Peng, C.C.; Chang, J.H.; Lin, H.Y.; Cheng, P.J.; Su, B.H. Intrauterine inflammation, infection, or both (Triple I): A new concept for chorioamnionitis. Pediatr. Neonatol. 2018, 59, 231–237.
- van Leeuwen, L.; Fourie, E.; van den Brink, G.; Bekker, V.; van Houten, M. Biomarkers for the diagnosis of early onset neonatal sepsis: A systematic review and meta-analysis. Submitted. 2022.
- Krueger, M.; Nauck, M.S.; Sang, S.; Hentschel, R.; Wieland, H.; Berner, R. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early-onset infection in premature infants. Neonatology 2001, 80, 118–123.
- Froeschle, G.M.; Bedke, T.; Boettcher, M.; Huber, S.; Singer, D.; Ebenebe, C.U. T cell cytokines in the diagnostic of early-onset sepsis. Pediatr. Res. 2021, 90, 191–196.
- Su, H.; Chang, S.S.; Han, C.M.; Wu, K.Y.; Li, M.C.; Huang, C.Y.; Lee, C.L.; Wu, J.Y.; Lee, C.C. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis-a systemic review and meta-analysis. J. Perinatol. 2014, 34, 268–274.
- Eichberger, J.; Resch, B. Reliability of Interleukin-6 Alone and in Combination for Diagnosis of Early Onset Neonatal Sepsis: Systematic Review. Front. Pediatr. 2022, 10, 840778.
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-Negative Early-Onset Neonatal Sepsis—At the Crossroad Between Efficient Sepsis Care and Antimicrobial Stewardship. Front. Pediatr. 2018, 6, 285.
- Kellogg, J.A.; Ferrentino, F.L.; Goodstein, M.H.; Liss, J.; Shapiro, S.L.; Bankert, D.A. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr. Infect. Dis. J. 1997, 16, 381–385.
- Garcia-Prats, J.A.; Cooper, T.R.; Schneider, V.F.; Stager, C.E.; Hansen, T.N. Rapid detection of microorganisms in blood cultures of newborn infants utilizing an automated blood culture system. Pediatrics 2000, 105, 523–527.
- Gude, S.S.; Peddi, N.C.; Vuppalapati, S.; Venu Gopal, S.; Marasandra Ramesh, H.; Gude, S.S. Biomarkers of Neonatal Sepsis: From Being Mere Numbers to Becoming Guiding Diagnostics. Cureus 2022, 14, e23215.
- Wang, J.; Wang, Z.; Zhang, M.; Lou, Z.; Deng, J.; Li, Q. Diagnostic value of mean platelet volume for neonatal sepsis: A systematic review and meta-analysis. Medicine 2020, 99, e21649.
- O’Reilly, D.; Murphy, C.A.; Drew, R.; El-Khuffash, A.; Maguire, P.B.; Ainle, F.N.; Mc Callion, N. Platelets in pediatric and neonatal sepsis: Novel mediators of the inflammatory cascade. Pediatr. Res. 2022, 91, 359–367.
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of Neonatal Sepsis: The Role of Inflammatory Markers. Front. Pediatr. 2022, 10, 840288.
- Hincu, M.A.; Zonda, G.I.; Stanciu, G.D.; Nemescu, D.; Paduraru, L. Relevance of Biomarkers Currently in Use or Research for Practical Diagnosis Approach of Neonatal Early-Onset Sepsis. Children 2020, 7, 309.
- Tiozzo, C.; Mukhopadhyay, S. Noninfectious influencers of early-onset sepsis biomarkers. Pediatr. Res. 2022, 91, 425–431.
- Dongen, O.R.E.; van Leeuwen, L.M.; de Groot, P.K.; Vollebregt, K.; Schiering, I.; Wevers, B.A.; Euser, S.M.; van Houten, M.A. Umbilical Cord Procalcitonin to Detect Early-Onset Sepsis in Newborns: A Promising Biomarker. Front. Pediatr. 2021, 9, 779663.
- Sinha, M.; Jupe, J.; Mack, H.; Coleman, T.P.; Lawrence, S.M.; Fraley, S.I. Emerging Technologies for Molecular Diagnosis of Sepsis. Clin. Microbiol. Rev. 2018, 31, e00089-17.
- Budding, A.E.; Hoogewerf, M.; Vandenbroucke-Grauls, C.M.; Savelkoul, P.H. Automated Broad-Range Molecular Detection of Bacteria in Clinical Samples. J. Clin. Microbiol. 2016, 54, 934–943.
- Escobar, G.J.; Puopolo, K.M.; Wi, S.; Turk, B.J.; Kuzniewicz, M.W.; Walsh, E.M.; Newman, T.B.; Zupancic, J.; Lieberman, E.; Draper, D. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics 2014, 133, 30–36.
- Puopolo, K.M.; Draper, D.; Wi, S.; Newman, T.B.; Zupancic, J.; Lieberman, E.; Smith, M.; Escobar, G.J. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics 2011, 128, e1155-63.
- Achten, N.B.; Klingenberg, C.; Benitz, W.E.; Stocker, M.; Schlapbach, L.J.; Giannoni, E.; Bokelaar, R.; Driessen, G.J.A.; Brodin, P.; Uthaya, S.; et al. Association of Use of the Neonatal Early-Onset Sepsis Calculator With Reduction in Antibiotic Therapy and Safety: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1032–1040.
- Snoek, L.; van Kassel, M.N.; Krommenhoek, J.F.; Achten, N.B.; Plotz, F.B.; van Sorge, N.M.; Brouwer, M.C.; van de Beek, D.; Bijlsma, M.W.; NOGBS Study Group. Neonatal early-onset infections: Comparing the sensitivity of the neonatal early-onset sepsis calculator to the Dutch and the updated NICE guidelines in an observational cohort of culture-positive cases. eClinicalMedicine 2022, 44, 101270.
- Morris, R.; Jones, S.; Banerjee, S.; Collinson, A.; Hagan, H.; Walsh, H.; Thornton, G.; Barnard, I.; Warren, C.; Reid, J.; et al. Comparison of the management recommendations of the Kaiser Permanente neonatal early-onset sepsis risk calculator (SRC) with NICE guideline CG149 in infants ≥34 weeks’ gestation who developed early-onset sepsis. Arch. Dis. Child Fetal. Neonatal. Ed. 2020, 105, 581–586.
- Benitz, W.E.; Achten, N.B. Technical assessment of the neonatal early-onset sepsis risk calculator. Lancet Infect. Dis. 2021, 21, e134–e140.
More
