Hydatid cyst is a common name for the larval stage of a tapeworm species of the genus Echinococcus granulosus, which is transmitted from animals to humans via the fecal–oral route.
Hydatid cysts predominantly affect the liver (75%), followed by the lung (15%), and they can affect many organs in the human body. Medical imaging modalities are the keystone for the diagnosis of hydatid cysts with high sensitivity and specificity. Ultrasound imaging with high resolution is the first choice for diagnosis, differential diagnosis, staging, establishing a role in interventional management, and follow-up, and it can differentiate Type I hydatid cysts from simple liver cysts. Unenhanced computed tomography (CT) is indicated where or when an ultrasound is unsatisfactory, such as with chest or brain hydatid cysts, when detecting calcification, and in obese patients. Magnetic resonance imaging (MRI) is superior for demonstrating cyst wall defects, biliary communication, neural involvement, and differentiating hydatid cysts from simple cysts using diffusion-weighted imaging (DWI) sequences. According to the phase of growth, hydatid cysts occur in different sizes and shapes, which may mimic benign or malignant neoplasms and may create diagnostic challenges in some cases. Hydatid cysts can mimic simple cysts, choledochal cysts, Caroli’s disease, or mesenchymal hamartomas of the liver. They can mimic lung cystic lesions, mycetoma, blood clots, Rasmussen aneurysms, and even lung carcinomas. Differential diagnosis can be difficult for arachnoid cysts, porencephalic cysts, pyogenic abscesses, and even cystic tumors of the brain, and can create diagnostic dilemmas in the musculoskeletal system.
- larval stage of Echinococcus granulosus
- unilocular simple cyst
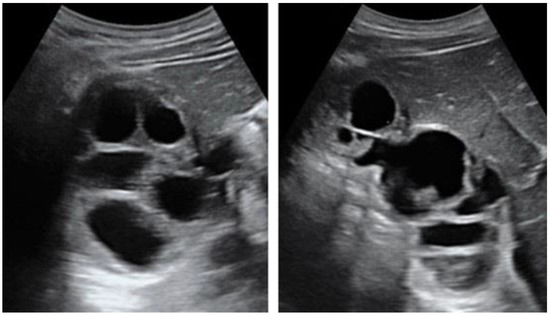
- cyst with floating membranes
1. Introduction
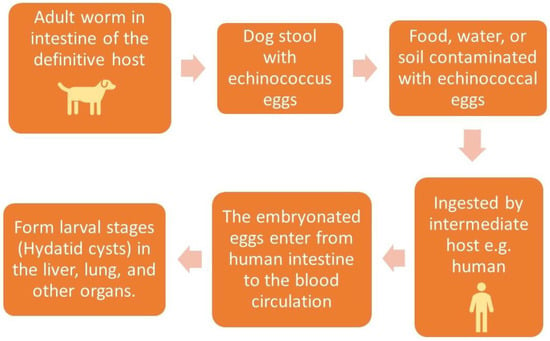
2. Diagnosis of Hydatid Cyst
Hydatid disease is diagnosed using medical imaging modalities, including abdominal ultrasound imaging, an X-ray of the chest, and computed tomography (CT) of the abdomen, chest, and brain. Serological antibody-detecting essays using diverse native antigens are only used to confirm the diagnosis because of the great difference in their sensitivities and specificities. An enzyme-linked immunosorbent assay (ELISA) using the synthetic peptide p176 has demonstrated a good performance in diagnosing hydatid disease, with up to 80% and 93% sensitivity and specificity, respectively [7]. Ultrasound imaging is the first choice due to its availability, lack of radiation, high resolution for diagnosis, differential diagnosis, staging, establishment of a role in interventional management, follow-up, and screening to assess the prevalence of abdominal hydatid cysts. Unenhanced CT is indicated where or when an ultrasound is unsatisfactory, such as in chest or brain hydatid cysts, when detecting calcification, and in obese patients [8]. Figure 3.
3. Structure of the Hydatid Cyst
Hydatid cysts have the following three layers: (1) The outer layer (pericyst) consists of modified host cells, including fibroblasts, giant cells, and eosinophils, forming a fibrous and protective zone. (2) The middle laminated acellular membrane, which allows passage of the nutrient. (3) The thin inner germinal layer. The middle laminated layer and the inner germinal layer form the true wall of the hydatid cyst, called the endocyst. The thickness of these layers tends to be the thickest in the liver compared to other organs. The acellular laminated layer is occasionally called an ectocyst [9]. The pericyst is also known as the ectocyst or adventitial layer [10]. The infectious embryonic tapeworm “scolices” develop from an outpouching of the germinal layer [9].4. Classification of Hydatid Cysts
I: The WHO Informal Working Group on Echinococcosis (WHO-IWGE) classification of hydatid cysts assigned six cyst stages into three clinical groups as follows: (1) The “active” group of developing cysts, which may be unilocular (CE1), or multivesicular with daughter vesicles (CE2), which are usually fertile cysts containing viable protoscoleces. (2) The “transitional” group, which may be cysts with a detachment of the endocyst membrane (CE3a), or predominantly solid cysts with daughter vesicles inside it (CE3b). (3) The “inactive” group includes solid contents (CE4), or solid contents with calcification (CE5), which are almost always nonviable. The WHO classification provides a rational basis for choosing the appropriate treatment scheme and follow-up [8,11][8][11]. Figure 4, Figure 5 and Figure 6.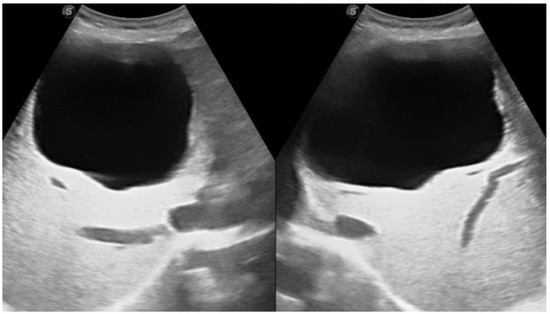
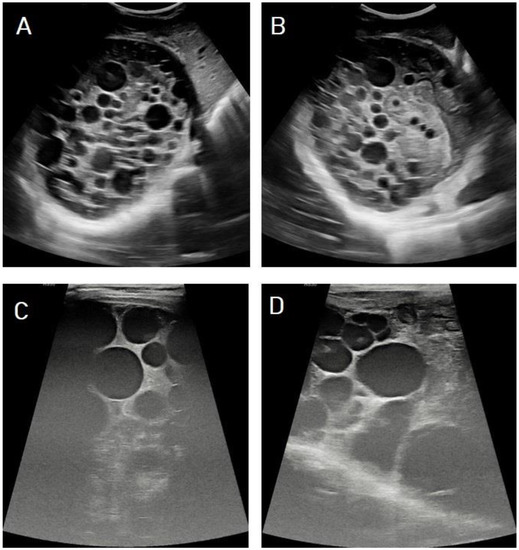
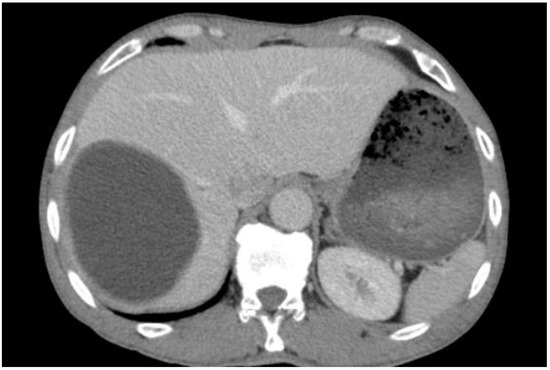
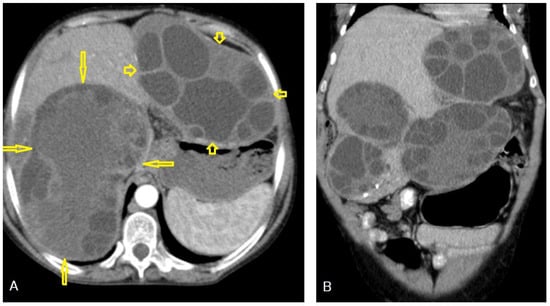
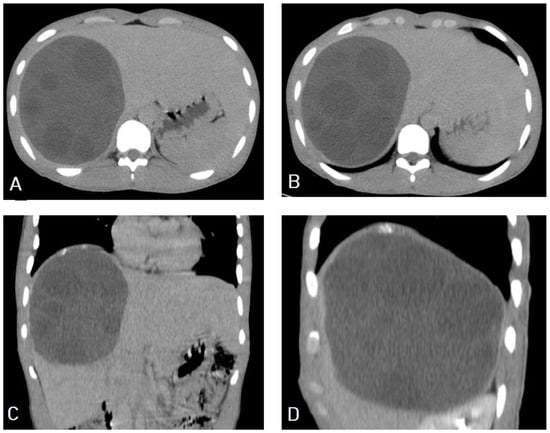
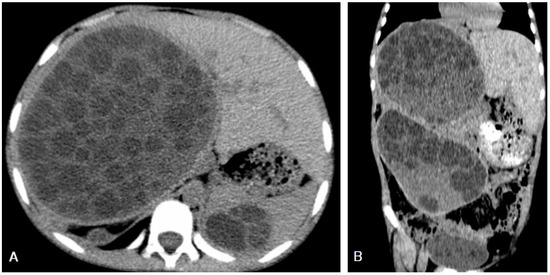
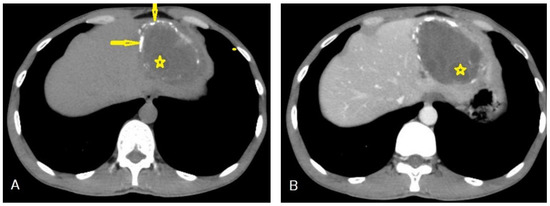
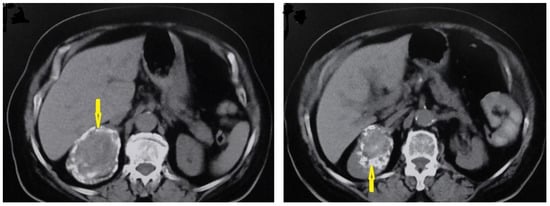
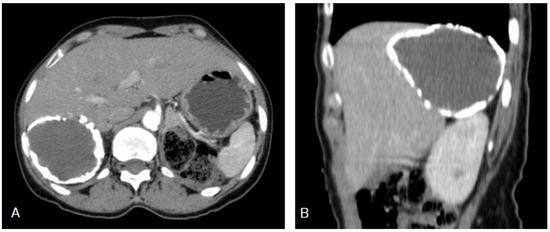
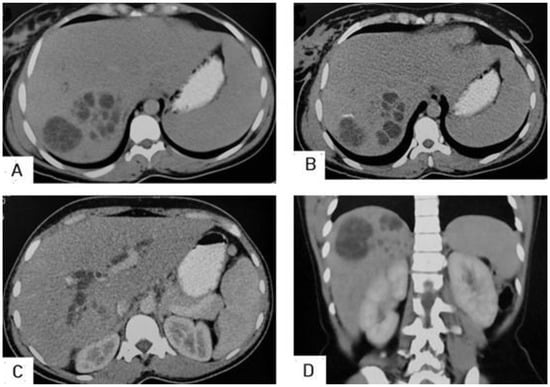
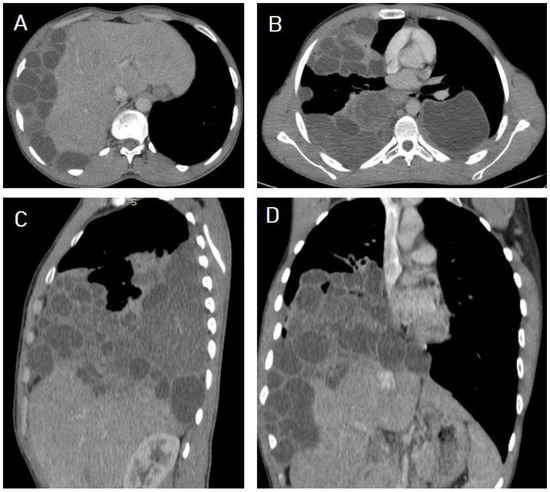
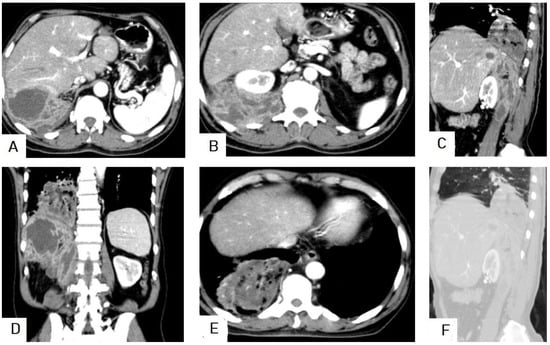
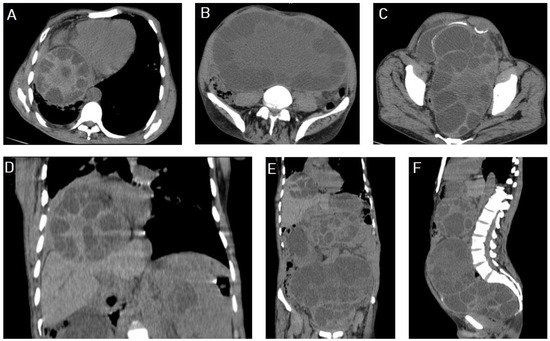
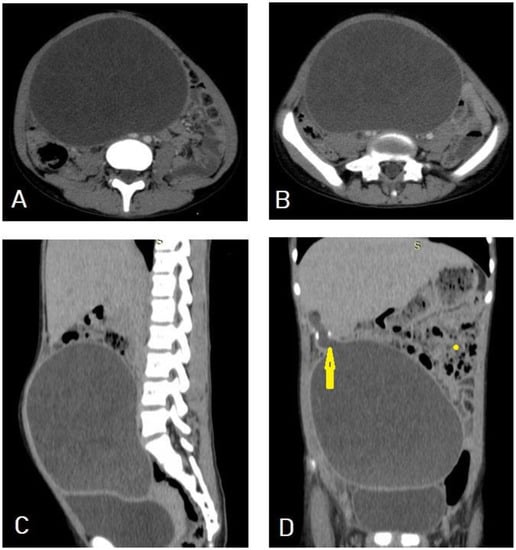
- Agudelo Higuita, N.I.; Brunetti, E.; McCloskey, C. Cystic Echinococcosis. Clin. Microbiol. 2016, 54, 518–523. https://doi.org/10.1128/JCM.02420-15.
- Zhang, T.; Li, B.; Liu, Y.; Liu, S. Risk Factors Associated With Echinococcosis in the General Chinese Population: A Meta-Analysis and Systematic Review. Public Health 2022, 10, 821265. https://doi.org/10.3389/fpubh.2022.821265.
- Lodhia, J.; Chugulu, S.; Sadiq, A.; Msuya, D.; Mremi, A. Giant isolated hydatid lung cyst: Two case reports. Med. Case Rep. 2020, 14, 200. https://doi.org/10.1186/s13256-020-02524-4.
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920. https://doi.org/10.1371/journal.pmed.1001920.
- Da Silva, A.M. Human echinococcosis: A neglected disease. Res. Pract. 2010, 2010, 583297. https://doi.org/10.1155/2010/583297.
- Ines, M.; Mariem, B.L.; Marwa, M.; Amina, B.S.; Chiraz, H. Isolated breast hydatid cyst: Imaging features. Case Rep. 2022, 10, e06362. https://doi.org/10.1002/ccr3.6362.
- Santivañez, S.J.; Arias, P.; Portocarrero, M.; Rodriguez, S.; Gonzalez, A.E.; Gilman, R.H.; Gavidia, C.M.; Garcia, H.H. Serological diagnosis of lung cystic hydatid disease using the synthetic p176 peptide. Vaccine Immunol. 2012, 19, 944–947. https://doi.org/10.1128/CVI.05540-11.
- Brunetti, E.; Tamarozzi, F.; Macpherson, C.; Filice, C.; Piontek, M.S.; Kabaalioglu, A.; Dong, Y.; Atkinson, N.; Richter, J.; Schreiber-Dietrich, D.; et al. Ultrasound and Cystic Echinococcosis. Ultrasound Int. Open. 2018, 4, E70–E78. https://doi.org/10.1055/a-0650-3807.
- Polat, P.; Atamanalp, S.S. Hepatic hydatid disease: Radiographics findings. Eurasian J. Med. 2009, 41, 49.
- Golzari, S.E.; Sokouti, M. Pericyst: The outermost layer of hydatid cyst. World J. Gastroenterol. 2014, 20, 1377–1378. https://doi.org/10.3748/wjg.v20.i5.1377.
- WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003, 85, 253–261. https://doi.org/10.1016/s0001-706x(02)00223-1.
- Mehta, P.; Prakash, M.; Khandelwal, N. Radiological manifestations of hydatid disease and its complications. Parasitol. 2016, 6, 103–112. https://doi.org/10.4103/2229-5070.190812.
- Marrone, G.; Crino, F.; Caruso, S.; Mamone, G.; Carollo, V.; Milazzo, M.; Gruttadauria, S.; Luca, A.; Gridelli, B. Multidisciplinary imaging of liver hydatidosis. World J. Gastroenterol. 2012, 18, 1438–1447. https://doi.org/10.3748/wjg.v18.i13.1438.
References
- Agudelo Higuita, N.I.; Brunetti, E.; McCloskey, C. Cystic Echinococcosis. J. Clin. Microbiol. 2016, 54, 518–523.
- Zhang, T.; Li, B.; Liu, Y.; Liu, S. Risk Factors Associated With Echinococcosis in the General Chinese Population: A Meta-Analysis and Systematic Review. Front. Public Health 2022, 10, 821265.
- Lodhia, J.; Chugulu, S.; Sadiq, A.; Msuya, D.; Mremi, A. Giant isolated hydatid lung cyst: Two case reports. J. Med. Case Rep. 2020, 14, 200.
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920.
- Da Silva, A.M. Human echinococcosis: A neglected disease. Gastroenterol. Res. Pract. 2010, 2010, 583297.
- Ines, M.; Mariem, B.L.; Marwa, M.; Amina, B.S.; Chiraz, H. Isolated breast hydatid cyst: Imaging features. Clin. Case Rep. 2022, 10, e06362.
- Santivañez, S.J.; Arias, P.; Portocarrero, M.; Rodriguez, S.; Gonzalez, A.E.; Gilman, R.H.; Gavidia, C.M.; Garcia, H.H. Serological diagnosis of lung cystic hydatid disease using the synthetic p176 peptide. Clin. Vaccine Immunol. 2012, 19, 944–947.
- Brunetti, E.; Tamarozzi, F.; Macpherson, C.; Filice, C.; Piontek, M.S.; Kabaalioglu, A.; Dong, Y.; Atkinson, N.; Richter, J.; Schreiber-Dietrich, D.; et al. Ultrasound and Cystic Echinococcosis. Ultrasound Int. Open. 2018, 4, E70–E78.
- Polat, P.; Atamanalp, S.S. Hepatic hydatid disease: Radiographics findings. Eurasian J. Med. 2009, 41, 49.
- Golzari, S.E.; Sokouti, M. Pericyst: The outermost layer of hydatid cyst. World J. Gastroenterol. 2014, 20, 1377–1378.
- WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003, 85, 253–261.
- Mehta, P.; Prakash, M.; Khandelwal, N. Radiological manifestations of hydatid disease and its complications. Trop. Parasitol. 2016, 6, 103–112.
- Marrone, G.; Crino, F.; Caruso, S.; Mamone, G.; Carollo, V.; Milazzo, M.; Gruttadauria, S.; Luca, A.; Gridelli, B. Multidisciplinary imaging of liver hydatidosis. World J. Gastroenterol. 2012, 18, 1438–1447.
