Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chang Lei and Version 2 by Peter Tang.
Bone regeneration is a comprehensive process that involves different stages, and various growth factors (GFs) play crucial roles in the entire process. GFs are widely used in clinical settings to promote bone repair; however, the direct application of GFs is often limited by their fast degradation and short local residual time. Additionally, GFs are expensive, and their use may carry risks of ectopic osteogenesis and potential tumor formation. Nanomaterials have shown great promise in delivering GFs for bone regeneration, as they can protect fragile GFs and control their release.
- nanomaterials
- growth factors
- bone regeneration
- bone defect
- bone graft
1. Introduction
The repairing of large bone defects remains a significant challenge in the clinic [1]. Over 2 million bone grafting surgeries are performed worldwide each year to restore the functions of defects [2], and the global market for bone grafting materials is expected to reach USD 1.4 billion by 2025 [3]. Among various grafting materials, autologous bone grafts have ideal physiologic properties and have been used for centuries [4]. Growth factors (GFs) that favor bone growth were also detected in fresh autologous bone grafts [5]; however, due to the limited resource of autologous bone and the risk of donor site morbidity [6], the development of synthetic bone graft substitutes for repairing bone is highly demanded. The ideal synthetic material should have good biocompatibility, excellent osteogenic as well as angiogenic activities, suitable mechanical properties, and an affordable cost [7]. With advances in material synthesis, many biomaterials have been developed as bone graft substitutes, including metals (iron, magnesium, alloy, etc.), ceramics (bioglass, calcium carbonate, gypsum, etc.) and polymers (collagen, chitosan, fibrin, etc.) [4][8][4,8]. Among them, biomaterials with nanostructures have attracted great interest in recent years as they enable the reassembly of new bone at the nanoscale, which is closer to the natural bone structure [9]. Specifically, the small nano-sizes, large surface areas, tunable surface chemistries, and porous structures of nanomaterials make them suitable for molecule (e.g., GFs and drugs) loading and delivery. Many nanomaterials have been used for bone regeneration, such as hydroxyapatite (HA) nanoparticles [10][11][12][13][14][10,11,12,13,14], silicon-based nanomaterials [15], carbon-based nanomaterials [16], graphene nanomaterials [17][18][17,18], and metal-based (gold, silver, platinum, iron, etc.) nanomaterials [19]. It is important to note that the nano–bio interactions that occur at the cellular, molecular, and atomic levels have significant implications for the safety and efficacy of nanomaterials in various biomedical applications, including drug delivery and tissue engineering.
Bone regeneration is a complex process that requires the coordination of different cells and signal pathways. It involves phases of inflammation, angiogenesis, and tissue healing [20]. As essential biomolecules present in all stages of bone regeneration, GFs stimulate osteogenesis by activating key genes and transcription factors or promoting osteoblasts’ differentiation [21]. In general, the whole process of bone regeneration is directly influenced by bone morphogenetic protein (BMP) [22] and transforming growth factor-beta (TGF-β) [23]. BMP-2, BMP-4, and BMP-7, from the BMP family, have been found to be osteogenic inducers [24], and the newly discovered BMP-9 has also attracted strong interest from scientists [25]. In the process of an inflammatory response, macrophages polarize to an M2 phenotype and release GFs, including interleukins (ILs) [26], fibroblast growth factors (FGFs) [27], and tumor necrosis factors (TNFs) [28], all of which have the function of inducing osteoblast migration [29]. During angiogenesis, vascular endothelial growth factor (VEGF) [30] and platelet-derived growth factor (PDGF) [31] play essential roles in the induction of the formation of new blood vessels. The main GFs involved in the tissue healing phase are FGFs and PDGF for fibroblast stimulation [32].
There are many studies that utilize GFs for bone defect treatment via either exogenous GF delivery or endogenous GF stimulation. It is difficult for exogenous GFs to function without a carrier due to the following reasons: The first is their short half-life [33], which requires repeated administration or an increased dosage to be overcome. The excessive use of BMP-2 has been shown to cause uncontrollable bone regeneration and cancer [34][35][34,35]. Although recombinant human bone morphogenetic proteins (rhBMPs) have been approved by the FDA for clinical use [36], the amount used in surgery is often a hundred times greater than that seen under normal conditions and could lead to uncontrollable consequences. Another critical reason is the biological instability under thermal or fluctuant pH conditions [37]. Without protection, in the specific microenvironment of defects natural GFs usually degrade rapidly and are unable to reach the cellular matrix, where they can function. Thirdly, controlled and targeted delivery is unachievable, which significantly hinders the function of GFs [38]. Therefore, using carriers to protect and achieve the controlled delivery of GFs is necessary [39]. As mentioned above, nanomaterials are popular carriers of GFs because of their unique nanoscale properties. The large surface areas and porous structures of nanomaterials facilitate the high loading of GFs, and their easy surface modification enables the targeted delivery of GFs. In turn, the addition of GFs can improve the osteogenic activity of nanomaterials. Therefore, nanomaterial-based exogenous GF delivery is a promising strategy for achieving satisfactory bone repairing outcomes.
Activating endogenous GFs to improve the body’s self-healing ability is another strategy. It usually induces lower immune rejection or resistance in patients compared to that of exogenous GF delivery. Nanomaterials are found to activate endogenous GFs via immune system stimulation, the stimulation of signal pathways, or gene therapy. The morphologies, surface chemistries, and contents of nanomaterials are found to modulate immune cells or signal pathways to release GFs for bone repairing [40][41][42][40,41,42]. Nanomaterials can also deliver genes and small-molecule drugs to stimulate the production of endogenous GFs and protect them from digestion [43].
Utilizing GFs in the treatment of bone repairing is an attractive area, and several reviews have been published in recent years that discuss either GF- or nanomaterial-based bone and tissue regeneration [44][45][46][47][44,45,46,47]. Here,In the researchersis review we discuss the integration of nanomaterials with GFs for bone regeneration, including exogenous GF delivery and endogenous GF activation (Figure 1). This resviearchw aims to elucidate the relationship between the properties of nanomaterials (Figure 2) and GFs in terms of bone regeneration, as well as providing an outlook on designing advanced nanomaterials with better GF utilization for bone defect treatment.
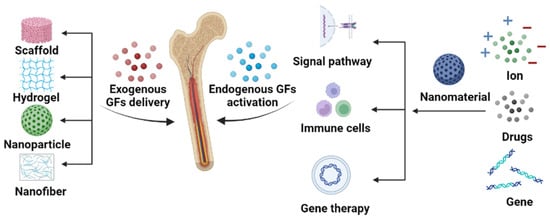
Figure 1. Schematic illustration of the synergistic effect of nanomaterials and GFs on bone repair. The roles of nanomaterials include exogenous GF delivery and endogenous GF stimulation.
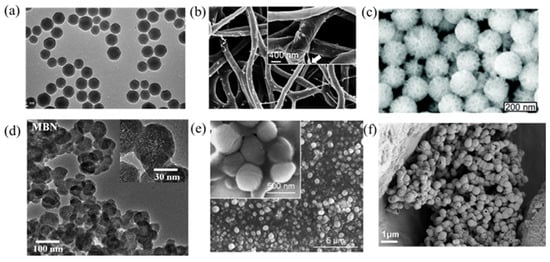
Figure 2. Images of various nanoparticles introduced here. (a) Mesoporous silica nanoparticles (MSNs) used for bFGF delivery [48]. (b) SF/PCL/PVA nanofibers used for dual GF delivery [49]. (c) Polyethylenimine-modified porous silica nanoparticles (PPSNs) used for pBMP-2 delivery [50]. (d) Magnetic iron oxide nanomaterials (MBNs) used for the stimulation of GF-related signal pathways [51]. (e) Deferoxamine@poly (ε-caprolactone) nanoparticles (DFO@PCL NP) used for the stimulation of macrophage polarization [52]. (f). RNA-activated matrices lipopolyplex (RAM-LPR) for miRNA delivery [53].
2. Exogenous Growth Factor Delivery
In the early days of GF therapy in bone defect treatment, GFs were often used via local direct injections, which were a very underutilized method due to the short half-life and unstable nature of GFs in human circulation [54]. To achieve the desired therapeutic effect, large doses of GFs were required, which led to high costs and severe side effects. Therefore, sustained release and local delivery strategies with which to increase the efficiency of GF utilization have become significant research areas in bone regeneration. As mentioned above, many nanomaterials have been developed as delivery platforms of GFs that are cost-effective and enable controlled release. GFs are usually immobilized to the surface of nanomaterials or loaded into their interior void/pores via physical adsorption or chemical bonding [45]. To date, the nanomaterials used for delivering GFs in bone regeneration have been molded into four forms: scaffolds, hydrogels, nanofibers, and nanoparticles (Figure 1).
3. Endogenous Growth Factor Activation
The use of nanomaterials for bone repair has been a topic of research for many years, with an emphasis on using exogenous GFs to achieve synergistic bone regeneration; however, with advancements in medical technology, the focus has shifted to the activation of endogenous GFs, which can avoid the potential immunological side effects associated with exogenous bioactive molecules. Studies have explored using a patient’s own endogenous GF-rich fibrin, combined with silica nanofibers, to form an injectable hydrogel that demonstrated sustainable GF release and promoted osteoblast differentiation [55]. Platelet-rich plasma, due to its rich GF content, has also been used to modify nanofibers [56]; however, there are many different types of GFs in blood, and not all of them have a positive effect on bone repair in addition to their clinical manifestations not being clear [57]. Scientists have therefore focused on bone substitute materials that stimulate the release of endogenous GFs, making the use of nanomaterials a major direction of research. Nanomaterials can stimulate the production of endogenous GFs by releasing ions, activating signal pathways or the immune system, or regulating GF expression levels in the body through genetic engineering (Figure 1).3.1. Signal Pathway Activation
Nanomaterials are increasingly being explored as a means by which to activate GFs in order to stimulate signal pathways and promote bone repair [42][58][59][42,58,59]. The release of ions or the loading of small-molecule drugs onto nanomaterials can activate GFs’ signal pathways, leading to enhanced bone regeneration. In recent years, studies have explored various types of nanomaterials and their impacts on endogenous GFs, demonstrating the potential for this approach to significantly improve bone repair outcomes. Overall, the use of nanomaterials to activate endogenous GFs and promote bone repair is an exciting area of research, with great potential to improve patient outcomes. The utilization of ions released from nanomaterials provides a unique and efficient way to stimulate signal pathways for GF production. This approach has demonstrated the potential to enhance cellular responses and promote bone regeneration. The use of nano-bioactive glass (nBG) has been shown to effectively release copper ions and stimulate the HIF-1α as well as TNF-α pathways, leading to an increase in the release of endogenous VEGF, angiogenin, IGF-1, and TIMP. A significant increase in the expression of VEGF, as well as the excellent osteoinductivity and osteoconductivity, are demonstrated in a rat cranial defect model [41]. A separate study on nBG showed that the release of Ca2+ and SiO44– from nBG stimulates the release of VEGF and promotes angiogenesis through the activation of the PI3K/Akt/HIF-1α pathway [60]. In addition, MSNs modified with strontium ions were found to effectively stimulate the Wnt pathway, leading to an increase in VEGF production and promoting both osteogenesis as well as angiogenesis [61]. Nanomaterials have also been used as the surface coatings of bone substitutes to activate pathways. For example, nGO was coated onto the surface of titanium implants and found to activate the FAK/P38 signal pathway, resulting in the increased osteogenic differentiation of BMSCs [62]. Similarly, nHA was employed as a coating on biphasic CaP scaffolds, which enhanced the expression of the BMP-2 gene via the activation of the BMP/Smad signal pathway [13]. Nanomaterials have the potential to enhance the release of endogenous GFs through the delivery of small-molecule drugs that activate signal pathways. One such example is the use of desferrioxamine (DFO), an iron chelator that creates a hypoxic environment and activates the HIF-1α signal pathway. In a study, DFO was loaded into polylactic acid (PLA) nanospheres and transformed into nanofibrous membranes. The results showed that the use of DFO consistently elevated HIF-1α mRNA expression [43]. To further improve the longevity of small-molecule drugs, a novel nano-scaffold was introduced. Specifically, a combination of DFO and GelMA was mixed and cross-linked on a bioglass scaffold functionalized with nanoclay (BG-XLS) to achieve the sustained release of DFO for up to 21 days. ELISA results revealed the high expression of two GFs, HIF-1α and VEGF, and endogenous bone regeneration was also observed in a rat cranial defect model [63]. Furthermore, nanomaterials have the potential to stimulate multiple signal pathways simultaneously through ion stimulation and loading with small-molecule drugs. A mesoporous bioglass nanoparticle (MBN) modified by Sr ions was loaded with phenamil, an activator of the BMP signal pathway. The combined effect of the Sr ions and phenamil accelerated the degradation of Smurf, a BMP pathway antagonist, leading to the upregulation of SMAD1/5/8, the stimulation of the BMP pathway, and an increase in the production of endogenous GFs [51]. Additionally, EVs secreted by genetically modified MSCs that consistently express the BMP-2 protein were used for bone repair. These EVs were shown to enhance the BMP-2 signal pathway of hMSCs, leading to the stimulation of the secretion of endogenous growth factors and resulting in bone regeneration in vivo in a rat cranial defect model [64].3.2. Immune System Stimulation
In the complex process of bone repair, a variety of cell types are involved, including the body’s immune cells, which play a crucial role in the initial inflammatory response that initiates the healing of the bone defect. Immune cells are capable of regulating bone homeostasis by producing various endogenous GFs, such as TGF-β and IL-4 [29], to stimulate bone repair. Hence, immune cells have become a target for researchers seeking to stimulate the production of endogenous GFs. It is worth noting that, upon entering the body, nanomaterials are often initially absorbed by the phagocytes of the immune system [65], providing a basis for their ability to stimulate the immune system. A variety of immune cells (e.g., macrophage, monocytes, and T cells) have been shown to activate and release endogenous GFs through ions carried by nanomaterials, small-molecule drugs, or the surface topology of materials.3.3. Gene Therapy
Gene therapy is an emerging therapeutic approach in molecular biology, aimed at treating or preventing genetic diseases by introducing or modifying genes in a patient’s cells. Gene therapy can be used to regulate gene expression in the body and treat a range of diseases. Gene therapy can also provide a long-term effect with a single administration, and has the potential to reduce or eliminate the need for repeated drug administration [66]. In the field of bone repair, gene therapies using synthetic RNAs and plasmids have been used to enhance the expression of osteogenic GFs in vivo; however, due to the degradation of these gene drugs by enzymes in the body and their inability to be effectively internalized by target cells [67], their use has been inefficient. To address this challenge, scientists are exploring the use of nanomaterials as carriers for gene drugs to protect them for safe and efficient transport into cells.- Verrier, S.; Alini, M.; Alsberg, E.; Buchman, S.R.; Kelly, D.; Laschke, M.W.; Menger, M.D.; Murphy, W.L.; Stegemann, J.P.; Schutz, M.; et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Cell Mater. 2016, 32, 87–110. https://doi.org/10.22203/ecm.v032a06.
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. https://doi.org/10.7759/cureus.17705.
- Research&Market. Global Cranial Implants Market (2019–2025)–Research and Markets. 2019. Available online: https://www.com/reports/4803302/global-cranial-implants-market-2019-2025 (
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Sci. Eng. C Mater. 2018, 82, 363–371. https://doi.org/10.1016/j.msec.2017.04.038.
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. Orthop. Trauma 2010, 24 (Suppl. S1), S36–S40. https://doi.org/10.1097/BOT.0b013e3181cec4a1.
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Health. Mater. 2018, 7, e1701061. https://doi.org/10.1002/adhm.201701061.
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? Clin. Periodontol. 2019, 46, 92–102. https://doi.org/10.1111/jcpe.13058.
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater Today 2010, 13, 24–30. https://doi.org/10.1016/S1369-7021(10)70014-6.
- Gong, T.; Xie, J.; Liao, J.F.; Zhang, T.; Lin, S.Y.; Lin, Y.F. Nanomaterials and bone regeneration. Bone Res. 2015, 3, https://doi.org/10.1038/boneres.2015.29.
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S.; et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. https://doi.org/10.1016/j.spinee.2021.01.019.
- Balagangadharan, K.; Chandran, S.V.; Arumugam, B.; Saravanan, S.; Venkatasubbu, G.D.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. J. Biol. Macromol. 2018, 111, 953–958. https://doi.org/10.1016/j.ijbiomac.2018.01.122.
- Casarrubios, L.; Gomez-Cerezo, N.; Sanchez-Salcedo, S.; Feito, M.J.; Serrano, M.C.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Diaz-Guemes, I.; Fernandez-Tome, B.; et al. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater. 2020, 101, 544–553. https://doi.org/10.1016/j.actbio.2019.10.033.
- Wang, J.; Wang, M.L.; Chen, F.Y.; Wei, Y.H.; Chen, X.N.; Zhou, Y.; Yang, X.; Zhu, X.D.; Tu, C.Q.; Zhang, X.D. Nano-Hydroxyapatite Coating Promotes Porous Calcium Phosphate Ceramic-Induced Osteogenesis Via BMP/Smad Signaling Pathway. J. Nanomed. 2019, 14, 7987–8000. https://doi.org/10.2147/Ijn.S216182.
- Yu, F.; Lian, R.X.; Liu, L.; Liu, T.; Bi, C.; Hong, K.; Zhang, S.Q.; Ren, J.Z.; Wang, H.K.; Ouyang, N.J.; et al. Biomimetic Hydroxyapatite Nanorods Promote Bone Regeneration via Accelerating Osteogenesis of BMSCs through T Cell-Derived IL-22. Acs Nano 2022, 16, 755–770. https://doi.org/10.1021/acsnano.1c08281.
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. https://doi.org/10.1016/j.actbio.2020.05.017.
- Peng, Z.L.; Zhao, T.S.; Zhou, Y.Q.; Li, S.H.; Li, J.J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Health. Mater. 2020, 9, e1901495. https://doi.org/10.1002/adhm.201901495.
- Shadjou, N.; Hasanzadeh, M.; Khalilzadeh, B. Graphene based scaffolds on bone tissue engineering. Bioengineered 2018, 9, 38–47. https://doi.org/10.1080/21655979.2017.1373539.
- Daneshmandi, L.; Barajaa, M.; Rad, A.T.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Health. Mater. 2021, 10, e202001414. https://doi.org/10.1002/adhm.202001414.
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Sci. 2022, 12, 6793. https://doi.org/10.3390/app12136793.
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. https://doi.org/10.1016/j.biomaterials.2018.07.017.
- Dhawan, U.; Jaffery, H.; Salmeron-Sanchez, M.; Dalby, M.J. An ossifying landscape: Materials and growth factor strategies for osteogenic signalling and bone regeneration. Opin. Biotechnol. 2022, 73, 355–363. https://doi.org/10.1016/j.copbio.2021.10.010.
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36 (Suppl. S3), S5–S7. https://doi.org/10.1016/j.injury.2005.07.027.
- Janssens, K.; ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming growth factor-beta1 to the bone. Rev. 2005, 26, 743–774. https://doi.org/10.1210/er.2004-0001.
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Rev. Endocrinol. 2016, 12, 203–221. https://doi.org/10.1038/nrendo.2016.12.
- Bharadwaz, A.; Jayasuriya, A.C. Osteogenic differentiation cues of the bone morphogenetic protein-9 (BMP-9) and its recent advances in bone tissue regeneration. Sci. Eng. C-Mater. 2021, 120, 111748. https://doi.org/10.1016/j.msec.2020.111748.
- Takeuchi, T.; Yoshida, H.; Tanaka, S. Role of interleukin-6 in bone destruction and bone repair in rheumatoid arthritis. Rev. 2021, 20, 102884. https://doi.org/10.1016/j.autrev.2021.102884.
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factor signaling in skeletal development and disease. Gene Dev. 2015, 29, 1463–1486. https://doi.org/10.1101/gad.266551.115.
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Natl. Acad. Sci. USA 2011, 108, 1585–1590. https://doi.org/10.1073/pnas.1018501108.
- Lee, J.; Byun, H.; Madhurakkat Perikamana, S.K.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Health. Mater. 2019, 8, e1801106. https://doi.org/10.1002/adhm.201801106.
- Pfeilschifter, J.; Oechsner, M.; Naumann, A.; Gronwald, R.G.; Minne, H.W.; Ziegler, R. Stimulation of bone matrix apposition in vitro by local growth factors: A comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology 1990, 127, 69–75. https://doi.org/10.1210/endo-127-1-69.
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. Bone Joint. Surg. Am. 2008, 90 (Suppl. S1), 48–54. https://doi.org/10.2106/JBJS.G.01231.
- Steed, D.L. The role of growth factors in wound healing. Clin. N. Am. 1997, 77, 575–586, https://doi.org/10.1016/s0039-6109(05)70569-7.
- Lauzon, M.A.; Daviau, A.; Marcos, B.; Faucheux, N. Nanoparticle-mediated growth factor delivery systems: A new way to treat Alzheimer’s disease. Control. Release 2015, 206, 187–205. https://doi.org/10.1016/j.jconrel.2015.03.024.
- Tian, H.; Zhao, J.; Brochmann, E.J.; Wang, J.C.; Murray, S.S. Bone morphogenetic protein-2 and tumor growth: Diverse effects and possibilities for therapy. Cytokine Growth Factor Rev. 2017, 34, 73–91. https://doi.org/10.1016/j.cytogfr.2017.01.002.
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer Risk After Use of Recombinant Bone Morphogenetic Protein-2 for Spinal Arthrodesis. Bone Jt. Surg. -Am. Vol. 2013, 95a, 1537–1545. https://doi.org/10.2106/Jbjs.L.01483.
- Lo, K.W.H.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Drug Deliver Rev. 2012, 64, 1277–1291. https://doi.org/10.1016/j.addr.2012.03.014.
- Kuroda, Y.; Kawai, T.; Goto, K.; Matsuda, S. Clinical application of injectable growth factor for bone regeneration: A systematic review. Regen. 2019, 39, 20. https://doi.org/10.1186/s41232-019-0109-x.
- Simpson, A.H.R.W.; Mills, L.; Noble, B. The role of growth factors and related agents in accelerating fracture healing. Bone Jt. Surg. Br. 2006, 88b, 701–705. https://doi.org/10.1302/0301-620x.88b6.
- Ordikhani, F.; Zandi, N.; Mazaheri, M.; Luther, G.A.; Ghovvati, M.; Akbarzadeh, A.; Annabi, N. Targeted nanomedicines for the treatment of bone disease and regeneration. Res. Rev. 2021, 41, 1221–1254. https://doi.org/10.1002/med.21759.
- Cui, Y.; Li, H.R.; Li, Y.X.; Mao, L.X. Novel insights into nanomaterials for immunomodulatory bone regeneration. Nanoscale Adv. 2022, 4, 334–352. https://doi.org/10.1039/d1na00741f.
- Dai, Q.Y.; Li, Q.T.; Gao, H.C.; Yao, L.T.; Lin, Z.F.; Li, D.G.; Zhu, S.L.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D printing of Cu-doped bioactive glass composite scaffolds promotes bone regeneration through activating the HIF-1 alpha and TNF-alpha pathway of hUVECs. Sci. 2021, 9, 5519–5532. https://doi.org/10.1039/d1bm00870f.
- Hu, M.; Xiao, F.; Ke, Q.F.; Li, Y.; Chen, X.D.; Guo, Y.P. Cerium-doped whitlockite nanohybrid scaffolds promote new bone regeneration via SMAD signaling pathway. Eng. J. 2019, 359, 1–12. https://doi.org/10.1016/j.cej.2018.11.116.
- Shi, R.; Zhang, J.S.; Niu, K.; Li, W.Y.; Jiang, N.; Li, J.L.; Yu, Q.S.; Wu, C.A. Electrospun artificial periosteum loaded with DFO contributes to osteogenesis via the TGF-beta 1/Smad2 pathway. Sci. 2021, 9, 2090–2102. https://doi.org/10.1039/d0bm01304h.
- Qu, M.Y.; Jiang, X.; Zhou, X.W.; Wang, O.R.; Wu, Q.Z.; Ren, L.; Zhu, J.X.; Zhu, S.S.; Tebon, P.; Sun, W.J.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Health. Mater. 2020, 9, e1901714. https://doi.org/10.1002/adhm.201901714.
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Biomater. 2018, 5, 197–211. https://doi.org/10.1093/rb/rby013.
- Niu, Y.; Wang, Z.; Shi, Y.; Dong, L.; Wang, C. Modulating macrophage activities to promote endogenous bone regeneration: Biological mechanisms and engineering approaches. Mater. 2021, 6, 244–261. https://doi.org/10.1016/j.bioactmat.2020.08.012.
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Bioeng. Biotechnol. 2020, 8, 922. https://doi.org/10.3389/fbioe.2020.00922.
- Shen, M.K.; Wang, L.L.; Feng, L.; Gao, Y.; Li, S.J.; Wu, Y.L.; Xu, C.Y.; Pei, G.X. bFGF-Loaded Mesoporous Silica Nanoparticles Promote Bone Regeneration Through the Wnt/?-Catenin Signalling Pathway. J. Nanomed. 2022, 17, 2593–2608. https://doi.org/10.2147/IJN.S366926InternationalJournalofNanomedicine2022.
- Cheng, G.; Yin, C.C.; Tu, H.; Jiang, S.; Wang, Q.; Zhou, X.; Xing, X.; Xie, C.Y.; Shi, X.W.; Du, Y.M.; et al. Controlled Co-delivery of Growth Factors through Layer-by-Layer Assembly of Core-Shell Nanofibers for Improving Bone Regeneration. Nano 2019, 13, 6372–6382. https://doi.org/10.1021/acsnano.8b06032.
- Xu, X.; Sun, M.; Wang, D.; Bu, W.; Wang, Z.; Shen, Y.; Zhang, K.; Zhou, D.; Yang, B.; Sun, H. Bone formation promoted by bone morphogenetic protein-2 plasmid-loaded porous silica nanoparticles with the involvement of autophagy. Nanoscale 2019, 11, 21953–21963. https://doi.org/10.1039/c9nr07017f.
- Lee, J.H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. https://doi.org/10.1016/j.actbio.2017.07.021.
- Zhang, J.; Tong, D.; Song, H.; Ruan, R.; Sun, Y.; Lin, Y.; Wang, J.; Hou, L.; Dai, J.; Ding, J.; et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Mater. 2022, 34, e2202044. https://doi.org/10.1002/adma.202202044.
- Wang, P.P.; Perche, F.; Midoux, P.; Cabral, C.S.D.; Malard, V.; Correia, I.J.; EI-Hafci, H.; Petite, H.; Logeart-Avramoglou, D.; Pichon, C. In Vivo bone tissue induction by freeze-dried collagen-nanohydroxyapatite matrix loaded with BMP2/NS1 mRNAs lipopolyplexes. Control. Release 2021, 334, 188–200. https://doi.org/10.1016/j.jconrel.2021.04.021.
- Azevedo, H.S.; Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Drug. Deliver. Rev. 2015, 94, 63–76. https://doi.org/10.1016/j.addr.2015.08.003.
- Ren, S.; Tang, X.; Liu, L.; Meng, F.; Yang, X.; Li, N.; Zhang, Z.; Aimaijiang, M.; Liu, M.; Liu, X.; et al. Reinforced Blood-Derived Protein Hydrogels Enable Dual-Level Regulation of Bio-Physiochemical Microenvironments for Personalized Bone Regeneration with Remarkable Enhanced Efficacy. Nano Lett. 2022, 22, 3904–3913. https://doi.org/10.1021/acs.nanolett.2c00057.
- Cheng, G.; Ma, X.; Li, J.M.; Cheng, Y.E.; Cao, Y.; Wang, Z.M.; Shi, X.W.; Du, Y.M.; Deng, H.B.; Li, Z.B. Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. J. Pharmaceut. 2018, 547, 656–666. https://doi.org/10.1016/j.ijpharm.2018.06.020.
- Miron, R.J.; Fujioka-Kobayashi, M.; Moraschini, V.; Zhang, Y.F.; Gruber, R.; Wang, H.L. Efficacy of platelet-rich fibrin on bone formation, part 1: Alveolar ridge preservation. J. Oral. Impl. 2021, 14, 181–194.
- Zheng, X.; Zhang, X.R.; Wang, Y.T.; Liu, Y.X.; Pan, Y.N.; Li, Y.J.; Ji, M.; Zhao, X.Q.; Huang, S.B.; Yao, Q.Q. Hypoxia-mimicking 3D bioglass-nanoclay scaffolds promote endogenous bone regeneration. Mater. 2021, 6, 3485–3495. https://doi.org/10.1016/j.bioactmat.2021.03.011.
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.N.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/beta-catenin signaling pathway. J. Nanomed. 2015, 10, 4383–4392. https://doi.org/10.2147/Ijn.S78775.
- Sanchez-Duffhues, G.; Hiepen, C.; Knaus, P.; ten Dijke, P. Bone morphogenetic protein signaling in bone homeostasis. Bone 2015, 80, 43–59. https://doi.org/10.1016/j.bone.2015.05.025.
- Yang, Z.; Yang, Z.Y.; Ding, L.; Zhang, P.; Liu, C.; Chen, D.F.; Zhao, F.J.; Wang, G.; Chen, X.F. Self-Adhesive Hydrogel Biomimetic Periosteum to Promote Critical-Size Bone Defect Repair via Synergistic Osteogenesis and Angiogenesis. ACS Appl. Mater. Interfaces 2022, 14, 36395–36410. https://doi.org/10.1021/acsami.2c08400.
- Liu, X.Z.; Sun, Y.; Shen, J.J.; Min, H.S.; Xu, J.; Chai, Y.M. Strontium doped mesoporous silica nanoparticles accelerate osteogenesis and angiogenesis in distraction osteogenesis by activation of Wnt pathway. -Nanotechnol. 2022, 41, 102496. https://doi.org/10.1016/j.nano.2021.102496.
- Li, Q.F.; Wang, Z.L. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. J. Nanomed. 2020, 15, 4659–4676. https://doi.org/10.2147/Ijn.S245608.
- Huang, C.C.; Kang, M.; Lu, Y.; Shirazi, S.; Diaz, J.I.; Cooper, L.F.; Gajendrareddy, P.; Ravindran, S. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. 2020, 109, 182–194. https://doi.org/10.1016/j.actbio.2020.04.017.
- Zolnik, B.S.; Gonzalez-Fernandez, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. https://doi.org/10.1210/en.2009-1082.
- Park, S.Y.; Kim, K.H.; Kim, S.; Lee, Y.M.; Seol, Y.J. BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry. Pharmaceutics 2019, 11, 393. https://doi.org/10.3390/pharmaceutics11080393.
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Ther. Methods Clin. Dev. 2016, 3, 16023. https://doi.org/10.1038/mtm.2016.23.
