Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Stacey N Doan.
Mounting evidence links obesity, metabolic dysfunction, mood, and cognition. Compromised metabolic health and psychological functioning worsen clinical outcomes, diminish quality of life, and contribute to comorbid conditions. As a medication with both insulin-sensitizing and anti-inflammatory effects, metformin affords the exciting opportunity to abrogate the bidirectional relationship between poor metabolic health and psychological function.
- obesity
- metabolic dysfunction
- mood
1. Introduction
With the burgeoning rise in obesity in the United States, an increasing number of Americans will face the deleterious health effects of excess weight and adiposity including Type 2 diabetes mellitus (T2DM), cardiovascular disease, and malignancies including colorectal, endometrial, and postmenopausal breast cancer [1,2][1][2]. Based on data from the National Health and Nutrition Examination Surveys (NHANES) from 1999–2000 through 2017–2018, the age-adjusted prevalence of obesity in U.S. adults increased from 30.5% to 42.4%; similarly, the age-adjusted prevalence of severe obesity rose from 4.7% to 9.2% [3]. For 2017–2018, middle-aged adults (40–59 years) had the highest prevalence of obesity at 44.8%; women had a higher prevalence of severe obesity (BMI ≥ 40 kg/m2) compared to men, at 11.5% versus 6.9% [3]. The prevalence of obesity was highest in non-Hispanic Black adults at 49.6%, followed by Hispanic (44.8%) and non-Hispanic White (42.2%) adults [3]. The steady rise in obesity over the past two decades is an urgent call to action.
Current strategies for weight loss include recommendations for healthy eating and regular exercise. While healthy eating and active living/exercise are effective strategies for managing obesity [4], engaging in healthy dietary behaviors and sustained physical activity is notoriously difficult. Although people who are overweight or have obesity are able to lose weight by short-term reports [5], NHANES data also indicate that most individuals are unable to maintain weight loss [6], and fewer adults who are overweight or have obesity report trying to lose weight [7]. This lack of adherence to a healthy lifestyle is due to multiple reasons, including (1) low tolerance to high-intensity exercise and higher perceived exertion in overweight individuals [8[8][9][10],9,10], and (2) difficulty in initiating and sustaining healthy lifestyle changes due to emotional and cognitive dysregulation integrally linked to obesity and overweight status [11,12][11][12]. Therefore, for people who are overweight or suffer from obesity, standard of care recommendations for lifestyle changes alone are likely to have little impact on reducing obesity risk. The difficulties encountered by many adults to reverse weight gain via recommended lifestyle practices of exercise and caloric restraint, despite the compelling incentive of health-related benefits, indicates a more complex problem.
2. Obesity and Metabolic Dysfunction
The chronic imbalance of energy intake versus energy expenditure leads to excessive fat accumulation and obesity. Obesity often leads to insulin resistance and changes in fuel utilization between carbohydrates, lipids, and protein, whereby insulin-stimulated glucose uptake by muscle and adipose tissue is impaired. Hyperinsulinemia ensues when cells become unresponsive to insulin, which fosters a proinflammatory milieu in adipose tissue with ectopic fat storage and aberrant energy usage [13,14][13][14]. Excess adiposity, especially visceral adiposity, also promotes chronic low-grade inflammation by macrophage, adipocyte–preadipocyte production of proinflammatory cytokines such as C-reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNFα) and adipokines such as leptin [15,16][15][16]; these endocrine effects of adipose tissue inflammation are considered causative of systemic inflammatory pathway activation and lead to insulin resistance. The co-occurrence of obesity/visceral adiposity, insulin resistance, dyslipidemia, and hypertension comprises metabolic syndrome [17[17][18],18], which carries increased risk for Type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD) [19], and postmenopausal breast cancer [20,21,22][20][21][22]. Obesity, proinflammatory signal transduction, and insulin resistance form a vicious cycle of dysregulated metabolism with deleterious health effects.3. Relations between Mood Dysregulation and Metabolic Dysfunction
Obesity and overweight status are closely associated with mood dysregulation in a codependent, bidirectional manner [23,24][23][24]. Obesity leads to a 25% increase in odds of mood and anxiety disorders [25]. The association between depression and obesity is more consistently evident among women than men [26]. Even after controlling for race and socioeconomic variables, young women with overweight status or obesity are more likely to report having sustained depressive mood than women who are lean [27]. Similarly, depression, as assessed by the Major Depression Inventory, is associated with increased risk of T2DM, as observed in population-based research [28]. Notably, depression is detected even at the prediabetes stage, with data demonstrating a 37–60% increase in prospective risk of developing T2DM among individuals with depression [29]. The relationship between depression and insulin resistance is observed even in individuals without abnormal or excessive fat accumulation. A prospective, longitudinal study showed that children with depression, measured with the Child Depression Inventory, develop insulin resistance independent of changes in BMI [30]. Consistent with these data, a recent meta-analysis suggests a small but significant association between depression and insulin resistance [31]. Note that meta-analyses showing an association between depressive symptoms or general distress and T2DM appear robust with both diagnostic (e.g., clinical records) and nondiagnostic (Centers for Epidemiologic Studies for Depression Scale, General Health Questionnaire) measures of depression [29]. Relatedly, even general negative affect, rather than diagnosed depression, is associated with metabolic health [32]. Higher negative affect or emotional distress (e.g., anxiety, depression, stress, sadness) [33] and lower positive affect or pleasant feelings or emotions (e.g., joy, calmness, interest, enthusiasm) [34], as assessed using the Positive and Negative Affect Schedule, are associated with increased BMI; for women, the effect is stronger [34]. Interestingly, the relationship between lower positive affect and higher BMI appeared to be explained by physical ill health [34]. Mood disorders such as depression are thought to lead to excessive weight gain because individuals with depression tend to have lowered energy and therefore are less physically active [35], and negative affect tends to be associated with higher intake of sweet, high-fat, and energy dense foods [36]; depression may also lead to use of food in an attempt to cope with emotional distress, given that food intake corresponds with acute physiological changes (e.g., increased serotonin) that can alleviate discomfort for the short term [37]. Moreover, analyses suggest that depression was more likely to precede obesity, rather than vice versa [38]. Additionally, pharmaceutical treatments for mood disorders can also induce weight gain [28[28][39][40][41],39,40,41], with potential to exacerbate insulin resistance and the inexorable cycle of mood and metabolic dysfunction [42,43][42][43].4. Relationship between Cognitive Function and Metabolic Dysfunction
In addition to mood, disruptions in cognition, specifically to higher level executive functioning, are observed among those with obesity [44]. Executive function (EF) is a set of higher-order cognitive processes necessary for goal-directed behavior, including working memory (i.e., short term storage of relevant, immediate information), inhibitory control (i.e., ability to control one’s attention, behavior, thoughts, and/or emotions to override impulsive or automatic/conditioned responses), and shifting/flexibility (i.e., ability to adapt behavior and thoughts to new, changing, or unexpected events) [45], as well as decision-making, which includes elements of applying and following rules [46], verbal fluency as a measure of ease or speed of semantic processing [47], and planning (i.e., forethought for future adaptive responses) [48]. EF skills are central for directing and guiding behavior, particularly in situations that are nonroutine or effortful, such as initiating health behavior change [49]. The majority of studies examining the relationship between obesity and EF have focused on differences between individuals with obesity and normal weight, with others focusing on overweight and normal weight individuals [44]. A meta-analysis conducted by Yang et al. [44] shows that a variety of EF tasks have been used to investigate the role of cognitive processes in excessive weight gain; inhibitory control and planning were the most common functions identified. Overall, results indicate EF deficits among overweight individuals or those with obesity. Specifically, individuals with obesity exhibited poor EF across all domains (i.e., working memory, inhibitory control, shifting/flexibility, decision-making, verbal fluency, planning), while overweight individuals only showed significant deficits in inhibitory control and working memory relative to normal weight controls [44]. Age, BMI, and sex did not seem to influence the pattern of results. Regarding working memory and decision-making, the type of task seemed to matter. For memory assessment, the digit span task and the delay discounting or Iowa gambling task exhibited larger effect sizes than the letter-numbering sequencing task. An important limitation identified by this meta-analysis, however, is the small number of studies focusing only on individuals who are overweight (rather than with obesity); lack of consideration of use of antiobesity medication and presence of a psychiatric disorder in many studies were also noted. Studies examining multiple measures of EF reveal inconsistent results regarding the relationship between EF deficits and excess adiposity/obesity. The Baltimore Longitudinal study of aging reported mixed results regarding type of cognitive task as well as the relationship between measures of adiposity and cognitive outcomes [50]. Study findings suggest that BMI and waist circumference were associated with poorer prospective memory; longitudinally, all three measures of adiposity (BMI, waist circumference, waist–hip ratio, or WHR as ratio of measurements of waist to hip circumference) demonstrated declining performance on the Benton Visual Retention test with increasing body size. While cross-sectional analyses showed that BMI was associated with significantly worse performance on the Letter and Category Fluency test, there were no longitudinal effects on any of the language measures (Letter and Category Fluency and Boston Naming) [50]. Regarding executive function (using the Trail Making B task, thought to be indicative of working memory and shifting abilities) [51], WHR was associated with slower performance over time. Surprisingly, however, obesity was associated with better attention and visuospatial ability. The relatively well-educated (average >16 years of education), and older (mean age 55.5, SD: 16.9) sample makes the results difficult to generalize to other populations. Results between adiposity and cognitive function were more consistent in a sample of middle-aged adults. The Framingham Heart study focused on BMI and WHR, both of which are risk factors for cardiovascular disease, in a sample of over 1800 men and women (age 40–69) at baseline. Results suggest that obesity and hypertension were related to worse executive function performance (using the Trails B test) [52]. The authors also found greater age-related cognitive decline in individuals with obesity and suggest the importance of controlling central obesity to reduce age-related cognitive declines. The relationship between BMI and cognition was further explored in the Whitehall II study [53], which assessed BMI over the life course at 25 years of age, during early midlife (mean age = 44), and late midlife (mean age = 61). Cognition was assessed in late midlife using the Mini Mental State Examination (MMSE), a screening measure of global cognition, and tests of short-term memory (a free recall task) and EF (reasoning and verbal fluency). Results suggest a curvilinear relationship, with both individuals who were underweight or with obesity having poorer cognition. Specifically, cumulative obesity (obesity at two or three time points) was associated with lower scores on the MMSE and measures of memory and EF; an increase in BMI from early to late midlife correlated with lower levels of executive function at late midlife [53]. In a review of 88 studies, Favieri et al. confirmed an inverse relation between obesity and a range of executive functioning measures (Wisconsin Card Sorting Test, Trail Making Test, Stroop Color-Word Task, Digit Span Test, Delay Discounting Task) [11]. These data suggest that obesity is a disorder of appetitive motivation, rather than simply a disorder of disruptions in homeostatic mechanisms of food intake. Dysfunction of the central melanocortin system, which is involved in regulation of energy homeostasis, food intake, satiety, and body weight, is also implicated in the pathogenesis of obesity [54,55][54][55]. The motivation to consume certain foods activates the mesolimbic dopamine system; cognitive functions (e.g., reward, desire) associated with the mesolimbic pathway are implicated in addiction [56]. At the same time, however, researchers have argued that these models need to incorporate EF processes, namely, those related to inhibitory control, which are localized in the prefrontal cortex [57]. Neurobiological and behavioral evidence suggests that individuals who have lower EF abilities are particularly susceptible to intake of high caloric foods, as well as weight gain [57].5. Mechanisms Linking Metabolic Health, Mood, and Cognitive Functioning
Researchers have theorized that both behavioral and biological mechanisms explain the relationships between mood, cognitive functioning, and metabolic health. From a behavioral perspective, depressed mood/negative affect leads to an increased preference for high-caloric food, which can serve as a form of emotion regulation [58]. Moreover, stress and negative affect can degrade higher executive functioning competencies that are critical to self-control. Finally, from a physiological perspective, long-term negative affect and/or chronic stress can lead to dysregulation in endocrine and immune systems that affect both brain and metabolic health, which can then, in turn, affect cognitive and mood disruptions and higher food intake, leading to a vicious, inescapable cycle. Observational studies of stress, using both life events as well as subjective distress scores [59], and lab-based manipulations of mood demonstrate that negative cues increase the salience of immediate, concrete goals, leading to preference for indulgent rather than healthy food [60]. Mood dysregulation is associated with poor health behaviors, including physical inactivity [61], binge eating [62], and increased caloric intake [63]. Relatedly, stress/negative affect [64] and depression [65] impair executive functioning abilities which are crucial to initiating and maintaining health behaviors. This is particularly problematic because EF is essential for behaviors (including health behaviors), which have an immediate cost in terms of time and effort but offer health benefits in the long term. Evidence suggests that the effects of mood/stress on health and health behaviors are mediated through disruptions in executive functioning abilities [66]. Prolonged depression and negative mood states involve activation of the hypothalamic–pituitary–adrenal axis, sympathoadrenal system, and proinflammatory cytokines [67]. Dysregulation in these systems can induce insulin resistance and contribute to diabetes risk [67]. A high-fat diet causes insulin resistance and T2DM by disrupting signaling circuits and neurotransmitter systems in the prefrontal cortex associated with motivation, reward, depression, and anxiety [68]. Insulin resistance is also associated with loss of motivation and heightened food-seeking behaviors thought to be mediated by differences in the anterior cingulate cortex—a known hippocampal motivational network that contributes to both depression and insulin resistance [69]. Evidence suggests that cortisol, the end-product of the hypothalamic–pituitary–adrenal axis (HPA), one of the main stress response systems, is positively associated with weight gain and enhanced secretion of proinflammatory hormones and cytokines (adipokines) by adipose tissue depots [70]. Proinflammatory cytokines, particularly IL-6 and CRP, have been implicated in both insulin resistance and T2DM [16]. Proinflammatory signaling may underlie depression in the setting of obesity and dysfunctional metabolism [71]. Obesity is also linked to mood dysregulation as a risk factor. Chronic inflammation arising from higher fat mass and metabolic dysfunction appears to be associated with psychological effects such as depression and anxiety [72]. In a study of data from a mental health questionnaire of participants in the U.K. Biobank (n = 145,668), Casanova et al. demonstrated that higher adiposity leads to higher odds of depression, severity of depression, and lower wellbeing, regardless of genetic predisposition to metabolic dysfunction (e.g., adiposity genetic variants with favorable or unfavorable metabolic profiles based on HDL cholesterol, triglycerides, and T2DM risk) [73]; limitations of the analysis include lack of diversity in the European study population and potential bias in the subset of participants involved in the mental health questionnaire substudy. Interestingly, the metabolically favorable adiposity variants were associated with higher levels of the proinflammatory cytokine CRP [73]. In sum, increasing evidence suggests the association of obesity and insulin resistance with depressed mood and cognitive dysfunction as an interrelated network that can become intransigent and bidirectionally entrained (Figure 1). Negative affect and stress bias individuals towards emotional responses, while at the same time degrading self-control abilities, leading to higher food consumption [74,75][74][75] and increasingly sedentary behaviors [76,77,78][76][77][78]. These behaviors over time can lead to reduced functioning in various quality of life domains, perpetuating, and possibly worsening, depressed mood. Relatedly, metabolic dysfunction including insulin resistance also appears to be associated with increases in mood disorders, suggesting bidirectional and convergent effects [23]. Higher BMI also decreases the effectiveness of antidepressants [79[79][80],80], and depression predicts unfavorable outcomes in a range of weight loss interventions, including surgical [81] and behavioral [82], as well as poor weight loss maintenance [83]. In fact, even nonclinical assessments of mood, such as stress (using the Perceived Stress Scale) [84[84][85],85], or primarily nondepressed individuals (BDI scores of <10) [86], show that higher levels predict lower efficacy of interventions, likely due to decreased engagement [84]. Studies also demonstrate that individuals who experience remission from depression are more likely to lose weight from lifestyle interventions than those who do not [86[86][87],87], perhaps due to higher levels of engagement in physical activity [88].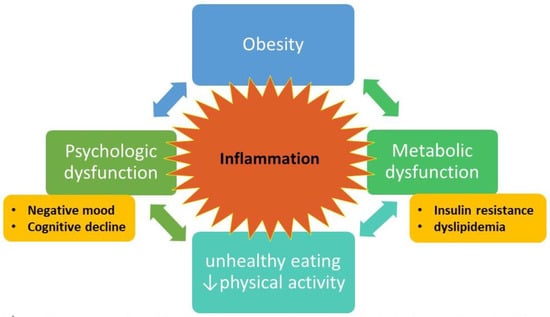
Figure 1. Conceptual model of obesity = metabolic and psychological dysfunction = inability to effect behavioral change to reverse obesity. Bidirectional arrows indicate feedback loops that form the intractable cycle. Inflammation is central to the model, with cytokines and other inflammatory factors fueling the bidirectional connections. Figure designed by L.D.Y. and S.D.
6. Metformin Provides an Exciting Pharmaceutical Intervention with Potential to Restore Metabolic Health while Simultaneously Improving Mood and Cognition
Metformin reduces insulin resistance associated with obesity. Approved in 1995 by the Food and Drug Administration for treatment of T2DM, metformin improves glucose tolerance via decreasing both hepatic production and intestinal absorption of glucose and increasing peripheral glucose uptake and utilization [100][89]. Metformin appears to lower fasting glucose via activation of AMP-kinase (AMPK), a pivotal molecule that redirects substrate utilization away from glucose and toward fatty acid beta oxidation. AMPK activation by metformin inhibits the mechanistic target of rapamycin (mTOR) in the liver with resultant downstream events including suppression of hepatic neogenesis [101][90]. Metformin crosses the blood–brain barrier and may elicit anti-inflammatory, neuroprotective effects [102,103][91][92]. In addition to the critical role of metformin in modulating metabolic and inflammation pathways that influence obesity, the drug’s well-established efficacy and safety profile for T2DM and prediabetes enable the possibility of novel repurposing. Below, we review the literature on the effects of metformin on mood and cognition in both animal and human populations.
References
- Walker, G.E.; Marzullo, P.; Ricotti, R.; Bona, G.; Prodam, F. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm. Mol. Biol. Clin. Investig. 2014, 19, 57–74.
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638.
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8.
- Khan, L.K.; Sobush, K.; Keener, D.; Goodman, K.; Lowry, A.; Kakietek, J.; Zaro, S. Recommended Community Strategies and Measurements to Prevent Obesity in the United States. MMWR Recommnedations Rep. 2009, 58, 1–29.
- Nicklas, J.M.; Huskey, K.W.; Davis, R.B.; Wee, C.C. Successful weight loss among obese U.S. adults. Am. J. Prev. Med. 2012, 42, 481–485.
- Kraschnewski, J.L.; Boan, J.; Esposito, J.; Sherwood, N.E.; Lehman, E.B.; Kephart, D.K.; Sciamanna, C.N. Long-term weight loss maintenance in the United States. Int. J. Obes. 2010, 34, 1644–1654.
- Snook, K.R.; Hansen, A.R.; Duke, C.H.; Finch, K.C.; Hackney, A.A.; Zhang, J. Change in Percentages of Adults with Overweight or Obesity Trying to Lose Weight, 1988–2014. JAMA 2017, 317, 971–973.
- Ekkekakis, P.; Lind, E. Exercise does not feel the same when you are overweight: The impact of self-selected and imposed intensity on affect and exertion. Int. J. Obes. 2006, 30, 652–660.
- Mattsson, E.; Larsson, U.E.; Rössner, S. Is walking for exercise too exhausting for obese women? Int. J. Obes. Relat. Metab. Disord. 1997, 21, 380–386.
- Hulens, M.; Vansant, G.; Claessens, A.L.; Lysens, R.; Muls, E. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scand. J. Med. Sci. Sport. 2003, 13, 98–105.
- Favieri, F.; Forte, G.; Casagrande, M. The Executive Functions in Overweight and Obesity: A Systematic Review of Neuro-psychological Cross-Sectional and Longitudinal Studies. Front. Psychol. 2019, 10, 2126.
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229.
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550.
- Pedersen, D.J.; Guilherme, A.; Danai, L.V.; Heyda, L.; Matevossian, A.; Cohen, J.; Nicoloro, S.M.; Straubhaar, J.; Noh, H.L.; Jung, D.; et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol. Metab. 2015, 4, 507–518.
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643.
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105.
- Parikh, R.M.; Mohan, V. Changing definitions of metabolic syndrome. Indian J. Endocrinol. Metab. 2012, 16, 7–12.
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237.
- Grundy, S.M. Obesity, Metabolic Syndrome, and Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600.
- Moore, L.L.; Chadid, S.; Singer, M.R.; Kreger, B.E.; Denis, G.V. Metabolic Health Reduces Risk of Obesity-Related Cancer in Framingham Study Adults. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2057–2065.
- Kabat, G.C.; Kim, M.Y.; Lee, J.S.; Ho, G.Y.; Going, S.B.; Beebe-Dimmer, J.; Manson, J.E.; Chlebowski, R.T.; Rohan, T.E. Metabolic Obesity Phenotypes and Risk of Breast Cancer in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1730–1735.
- Park, Y.-M.M.; White, A.J.; Nichols, H.B.; O’brien, K.M.; Weinberg, C.R.; Sandler, D.P. The association between metabolic health, obesity phenotype and the risk of breast cancer. Int. J. Cancer 2017, 140, 2657–2666.
- Mansur, R.B.; Brietzke, E.; McIntyre, R.S. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci. Biobehav. Rev. 2015, 52, 89–104.
- McElroy, S.L.; Kotwal, R.; Malhotra, S.; Nelson, E.B.; Keck, P.E., Jr.; Nemeroff, C.B. Are mood disorders and obesity related? A review for the mental health professional. J. Clin. Psychiatry 2004, 65, 634–651.
- Simon, G.E.; Von Korff, M.; Saunders, K.; Miglioretti, D.L.; Crane, P.K.; van Belle, G.; Kessler, R.C. Association Between Obesity and Psychiatric Disorders in the US Adult Population. Arch. Gen. Psychiatry 2006, 63, 824–830.
- de Wit, L.; Luppino, F.; van Straten, A.; Penninx, B.; Zitman, F.; Cuijpers, P. Depression and obesity: A meta-analysis of community-based studies. Psychiatry Res. 2010, 178, 230–235.
- Heo, M.; Pietrobelli, A.; Fontaine, K.R.; Sirey, A.J.; Faith, M.S. Depressive mood and obesity in US adults: Comparison and moderation by sex, age, and race. Int. J. Obes. 2005, 30, 513–519.
- Deleskog, A.; Ljung, R.; Forsell, Y.; Nevriana, A.; Almas, A.; Möller, J. Severity of depression, anxious distress and the risk of type 2 diabetes—A population-based cohort study in Sweden. BMC Public Health 2019, 19, 1174.
- Mezuk, B.; Eaton, W.W.; Albrecht, S.; Golden, S.H. Depression and Type 2 Diabetes Over the Lifespan. Diabetes Care 2008, 31, 2383–2390.
- Shomaker, L.B.; Tanofsky-Kraff, M.; Stern, E.A.; Miller, R.; Zocca, J.M.; Field, S.E.; Yanovski, S.Z.; Hubbard, V.S.; Yanovski, J.A. Longitudinal Study of Depressive Symptoms and Progression of Insulin Resistance in Youth at Risk for Adult Obesity. Diabetes Care 2011, 34, 2458–2463.
- Kan, C.; Silva, N.; Golden, S.H.; Rajala, U.; Timonen, M.; Stahl, D.; Ismail, K. A Systematic Review and Meta-analysis of the Association Between Depression and Insulin Resistance. Diabetes Care 2013, 36, 480–489.
- Carr, D.; Friedman, M.A.; Jaffe, K. Understanding the relationship between obesity and positive and negative affect: The role of psychosocial mechanisms. Body Image 2007, 4, 165–177.
- Pasco, A.J.; Williams, L.; Jacka, F.N.; Brennan-Olsen, S.; Berk, M. Obesity and the relationship with positive and negative affect. Aust. N. Z. J. Psychiatry 2013, 47, 477–482.
- Jorm, A.F.; Korten, A.E.; Christensen, H.; Jacomb, P.A.; Rodgers, B.; Parslow, R.A. Association of obesity with anxiety, depression and emotional well-being: A community survey. Aust. N. Z. J. Public Health 2003, 27, 434–440.
- Roshanaei-Moghaddam, B.; Katon, W.J.; Russo, J. The longitudinal effects of depression on physical activity. Gen. Hosp. Psychiatry 2009, 31, 306–315.
- Oliver, G.; Wardle, J.; Gibson, E.L. Stress and Food Choice: A Laboratory Study. Psychosom. Med. 2000, 62, 853–865.
- Hryhorczuk, C.; Sharma, S.; Fulton, S.E. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 2013, 7, 177.
- Fabricatore, A.N.; Wadden, T.A. Psychological aspects of obesity. Clin. Dermatol. 2004, 22, 332–337.
- El Asmar, K.; Fève, B.; Colle, R.; Trabado, S.; Verstuyft, C.; Gressier, F.; Vievard, A.; Haffen, E.; Polosan, M.; Ferreri, F.; et al. Early weight gain predicts later metabolic syndrome in depressed patients treated with antidepressants: Findings from the METADAP cohort. J. Psychiatr. Res. 2018, 107, 120–127.
- Newcomer, J.W.; Eriksson, H.; Zhang, P.; Meehan, S.R.; Weiss, C. Changes in Metabolic Parameters and Body Weight in Patients with Major Depressive Disorder Treated With Adjunctive Brexpiprazole: Pooled Analysis of Phase 3 Clinical Studies. J. Clin. Psychiatry 2019, 80, 2120.
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288.
- Woo, Y.S.; Seo, H.-J.; McIntyre, R.S.; Bahk, W.-M. Obesity and Its Potential Effects on Antidepressant Treatment Outcomes in Patients with Depressive Disorders: A Literature Review. Int. J. Mol. Sci. 2016, 17, 80.
- Puzhko, S.; Aboushawareb, S.A.; Kudrina, I.; Schuster, T.; Barnett, T.A.; Renoux, C.; Bartlett, G. Excess body weight as a predictor of response to treatment with antidepressants in patients with depressive disorder. J. Affect. Disord. 2020, 267, 153–170.
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244.
- Carlson, S.M.; Zelazo, P.D.; Faja, S. Executive function. In Oxford Library of Psychology. The Oxford Handbook of Developmental Psychology: Body and Mind; Zelazo, P.D., Ed.; Oxford University Press: Oxford, UK, 2013; Volume 1, pp. 706–743.
- Rangel, A.; Camerer, C.; Montague, P.R. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008, 9, 545–556.
- Troyer, A.K.; Moscovitch, M.; Winocur, G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology 1997, 11, 138–146.
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Fischer, J.S. Neuropsychological Assessment; Oxford University Press: Oxford, UK, 2004.
- Banich, M.T. Executive Function: The Search for an Integrated Account. Curr. Dir. Psychol. Sci. 2009, 18, 89–94.
- Gunstad, J.; Lhotsky, A.; Wendell, C.R.; Ferrucci, L.; Zonderman, A.B. Longitudinal Examination of Obesity and Cognitive Function: Results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology 2010, 34, 222–229.
- Sánchez-Cubillo, I.; Periáñez, J.; Adrover-Roig, D.; Rodríguez-Sánchez, J.; Ríos-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450.
- Wolf, P.; Beiser, A.; Elias, M.; Au, R.; Vasan, R.; Seshadri, S. Relation of Obesity to Cognitive Function: Importance of Central Obesity and Synergistic Influence of Concomitant Hypertension. The Framingham Heart Study. Curr. Alzheimer Res. 2007, 4, 111–116.
- Sabia, S.; Kivimaki, M.; Shipley, M.J.; Marmot, M.G.; Singh-Manoux, M. Body mass index over the adult life course and cognition in late midlife: The Whitehall II Cohort Study. Am. J. Clin. Nutr. 2009, 89, 601–607.
- Goit, R.K.; Taylor, A.W.; Lo, A.C.Y. The central melanocortin system as a treatment target for obesity and diabetes: A brief overview. Eur. J. Pharmacol. 2022, 924, 174956.
- Micioni Di Bonaventura, E.; Botticelli, L.; Tomassoni, D.; Tayebati, S.K.; Micioni Di Bonaventura, M.V.; Cifani, C. The Melanocortin System behind the Dysfunctional Eating Behaviors. Nutrients 2020, 12, 3502.
- Goudriaan, A.E.; Oosterlaan, J.; de Beurs, E.; Van den Brink, W. Pathological gambling: A comprehensive review of biobehavioral findings. Neurosci. Biobehav. Rev. 2004, 28, 123–141.
- Appelhans, B.M. Neurobehavioral Inhibition of Reward-driven Feeding: Implications for Dieting and Obesity. Obesity 2009, 17, 640–647.
- Singh, M. Mood, food, and obesity. Front. Psychol. 2014, 5, 925.
- Kandiah, J.; Yake, M.; Jones, J.; Meyer, M. Stress influences appetite and comfort food preferences in college women. Nutr. Res. 2006, 26, 118–123.
- Gardner, M.P.; Wansink, B.; Kim, J.; Park, S. Better moods for better eating?: How mood influences food choice. J. Consum. Psychol. 2014, 24, 320–335.
- Poole, L.; Steptoe, A.; Wawrzyniak, A.J.; Bostock, S.; Mitchell, E.; Hamer, M. Associations of objectively measured physical activity with daily mood ratings and psychophysiological stress responses in women. Psychophysiology 2011, 48, 1165–1172.
- Wegner, K.E.; Smyth, J.M.; Crosby, R.D.; Wittrock, D.; Wonderlich, S.A.; Mitchell, J.E. An evaluation of the relationship between mood and binge eating in the natural environment using ecological momentary assessment. Int. J. Eat. Disord. 2002, 32, 352–361.
- Wurtman, J.; Wurtman, R. The Trajectory from Mood to Obesity. Curr. Obes. Rep. 2018, 7, 1–5.
- Plieger, T.; Reuter, M. Stress & executive functioning: A review considering moderating factors. Neurobiol. Learn. Mem. 2020, 173, 107254.
- Snyder, H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol. Bull. 2013, 139, 81–132.
- O’Neill, J.; Kamper-DeMarco, K.; Chen, X.; Orom, H. Too stressed to self-regulate? Associations between stress, self-reported executive function, disinhibited eating, and BMI in women. Eat. Behav. 2020, 39, 101417.
- Golden, S.H. A Review of the Evidence for a Neuroendocrine Link Between Stress, Depression and Diabetes Mellitus. Curr. Diabetes Rev. 2007, 3, 252–259.
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249.
- Singh, M.K.; Leslie, S.M.; Packer, M.M.; Zaiko, Y.V.; Phillips, O.R.; Weisman, E.F.; Wall, D.M.; Jo, B.; Rasgon, N. Brain and behavioral correlates of insulin resistance in youth with depression and obesity. Horm. Behav. 2019, 108, 73–83.
- Bose, M.; Oliván, B.; Laferrère, B. Stress and obesity: The role of the hypothalamic–pituitary–adrenal axis in metabolic disease. Curr. Opin. Endocrinol. Diabetes 2009, 16, 340–346.
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry 2013, 70, 31–41.
- Mann, J.N.; Thakore, J.H. Melancholic Depression and Abdominal Fat Distribution: A Mini-Review. Stress 1999, 3, 1–15.
- Casanova, F.; O’Loughlin, J.; Martin, S.; Beaumont, R.N.; Wood, A.R.; Watkins, E.R.; Freathy, R.M.; Hagenaars, S.P.; Frayling, T.M.; Yaghootkar, H.; et al. Higher adiposity and mental health: Causal inference using Mendelian randomization. Hum. Mol. Genet. 2021, 30, 2371–2382.
- Dallman, M.F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010, 21, 159–165.
- Groesz, L.M.; McCoy, S.; Carl, J.; Saslow, L.; Stewart, J.; Adler, N.; Laraia, B.; Epel, E. What is eating you? Stress and the drive to eat. Appetite 2011, 58, 717–721.
- Ng, D.M.; Jeffery, R.W. Relationships Between Perceived Stress and Health Behaviors in a Sample of Working Adults. Health Psychol. 2003, 22, 638–642.
- Boutelle, K.N.; Murray, D.M.; Jeffery, R.W.; Hennrikus, D.J.; Lando, H.A. Associations between Exercise and Health Behaviors in a Community Sample of Working Adults. Prev. Med. 2000, 30, 217–224.
- Stetson, B.A.; Rahn, J.M.; Dubbert, P.M.; Wilner, B.J.; Mercury, M.G. Prospective evaluation of the effects of stress on exercise adherence in community-residing women. Health Psychol. 1997, 16, 515–520.
- Kloiber, S.; Ising, M.; Reppermund, S.; Horstmann, S.; Dose, T.; Majer, M.; Zihl, J.; Pfister, H.; Unschuld, P.G.; Holsboer, F.; et al. Overweight and Obesity Affect Treatment Response in Major Depression. Biol. Psychiatry 2007, 62, 321–326.
- Oskooilar, N.; Wilcox, C.S.; Tong, M.-L.; Grosz, D.E. Body Mass Index and Response to Antidepressants in Depressed Research Subjects. J. Clin. Psychiatry 2009, 70, 1609–1610.
- Legenbauer, T.; Petrak, F.; de Zwaan, M.; Herpertz, S. Influence of depressive and eating disorders on short- and long-term course of weight after surgical and nonsurgical weight loss treatment. Compr. Psychiatry 2011, 52, 301–311.
- Somerset, S.; Graham, L.; Markwell, K. Depression scores predict adherence in a dietary weight loss intervention trial. Clin. Nutr. 2011, 30, 593–598.
- Ohsiek, S.; Williams, M. Psychological factors influencing weight loss maintenance: An integrative literature review. J. Am. Acad. Nurse Pract. 2011, 23, 592–601.
- Trief, P.M.; Cibula, D.; Delahanty, L.M.; Weinstock, R.S. Depression, stress, and weight loss in individuals with metabolic syndrome in SHINE, a DPP translation study. Obesity 2014, 22, 2532–2538.
- Elder, C.R.; Gullion, C.M.; Funk, K.L.; DeBar, L.L.; Lindberg, N.M.; Stevens, V.J. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int. J. Obes. 2011, 36, 86–92.
- Faulconbridge, L.F.; Wadden, T.A.; Rubin, R.R.; Wing, R.R.; Walkup, M.P.; Fabricatore, A.N.; Coday, M.; Van Dorsten, B.; Mount, D.L.; Ewing, L.J.; et al. One-Year Changes in Symptoms of Depression and Weight in Overweight/Obese Individuals with Type 2 Diabetes in the Look AHEAD Study. Obesity 2012, 20, 783–793.
- Pagoto, S.; Schneider, K.L.; Whited, M.C.; Oleski, J.L.; Merriam, P.; Appelhans, B.; Ma, Y.; Olendzki, B.; Waring, E.M.; Busch, A.M.; et al. Randomized controlled trial of behavioral treatment for comorbid obesity and depression in women: The Be Active Trial. Int. J. Obes. 2013, 37, 1427–1434.
- Simon, G.E.; Rohde, P.; Ludman, E.J.; Jeffery, R.W.; Linde, J.A.; Operskalski, B.H.; Arterburn, D. Association between change in depression and change in weight among women enrolled in weight loss treatment. Gen. Hosp. Psychiatry 2010, 32, 583–589.
- Glucophage Package Insert; Bristol-Myers Squibb Company: Princeton, NJ, USA, 2018.
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471.
- Labuzek, K.; Liber, S.; Gabryel, B.; Adamczyk, J.; Okopieñ, B. Metformin increases phagocytosis and acidifies lysosomal/endosomal compartments in AMPK-dependent manner in rat primary microglia. Naunyn Schmiedebergs Arch. Pharm. 2010, 381, 171–186.
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965.
More
