Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Xi Yang and Version 3 by Lindsay Dong.
Regulatory T cells (Tregs) and T helper 17 cells (Th17) are two CD4+ T cell subsets with antagonist effects. Th17 cells promote inflammation, whereas Tregs are crucial in maintaining immune homeostasis. Th17 cells and Treg cells are the foremost players in several inflammatory diseases.
- Th17 cells
- Treg cells
- inflammation
- lung
1. Introduction
The immune system acts as the guardian of the host and functions to defend against foreign antigens, induce self-tolerance, and promote immunological memory. However, it is not protective or beneficial all the time. The individual’s tissue components may be attacked by the immunological reaction resulting in autoimmune diseases in specific settings. It is certain that a single theory or mechanism cannot explain autoimmune diseases. As proposed by Shoenfeld and Isenberg, autoimmune diseases are caused by various factors, including immunological, genetic, hormonal, and environmental factors [1]. Non-genetic components rather than inherent components play a dominant role in determining disease susceptibility and severity, which has been demonstrated by the discordance of autoimmune diseases in identical twins [2]. Immunological factors play a vital role in the initiation, progression, and regression of autoimmune diseases. In a typical setting, T cells are tolerant to physiological levels of self-antigen. However, this state of tolerance breaks down in some individuals, resulting in autoimmune/inflammatory diseases. One of the critical features of inflammatory diseases is the deregulated Th1/T helper 17 cells (Th17) 17 responses, frequently accompanied by a reduction and/or alteration of regulatory T (Treg) cells. Th17 cells serve as inflammatory cells, which in excess, promote inflammatory diseases. On the other hand, Treg cells show suppressor function, which, when in failure, contributes to the same disease [3].
1.1. Th17 Cells
Initial studies by Infante-Duarte et al. identified CD4+ T cells producing IL-17A as a T helper cell subset distinct from Th1 and Th2 cell subsets [4]. This subset, called Th17 cells, predominantly produces interleukin-17A (IL-17A), IL-17F, IL-21, and IL-22 [5]. IL-17A, originally named CTLA8, was cloned and described by Rouvier et al. [6]. It is a homodimeric glycoprotein with 155 amino acids linked by disulfide bonds. IL-17F, also produced by Th17 cells, shows 55% similarity with IL-17A, and they form IL-17F homodimers, IL-17A homodimers, or IL-17A-IL-17F heterodimers [7]. IL-17 binds to its receptor (IL-17R), a transmembrane protein, highly expressed in rats and mice’s spleen, kidneys, liver, and lungs [8]. Th17 cells require the transcription factor, RORγt, and cytokine IL-6 in combination with transforming growth factor-β (TGF-β) for their differentiation [9]. IL-6 acts as a major factor guiding the differentiation of T cells into Th17 cells or Treg cells [9]. IL-21, together with TGF-β, also functions as an alternative pathway to generate Th17 cells [10]. Once they reach the site of inflammation, IL-17 released by Th17 cells stimulates the expression of pro-inflammatory cytokines like granulocyte-macrophage colony-stimulating factor, Granulocyte-colony stimulating factor, IL-6 and tumor necrosis factor-alpha (TNF-α) [11]. In addition, IL-17 also promotes the secretion of CXC chemokines, which attracts neutrophils in vivo [11]. Moreover, IL-17 stimulates the production of antimicrobial peptides, such as β-defensin and S100 proteins, providing defense against a wide range of microorganisms [12][13][12,13].
1.2. Treg Cells
As the bias towards pro-inflammatory cytokines and cells induces the development and perpetuation of autoimmunity, immunoregulatory factors are thought to straighten out the laterality. Regulatory T cells are crucial members of the family of immunoregulatory cells that preserve self-tolerance and fine-tune the immune response. Treg cells suppress inflammation by cell-cell contact or releasing cytokines, such as IL-10 or TGF-β, and they require the transcription factor FoxP3 for their differentiation [3][14][3,19]. In recent years, research has identified two types of Treg cells called natural Treg cells (nTreg) and inducible Treg cells (iTreg). nTreg cells develop in the thymus, and when entering peripheral tissues, they suppress self-reactive T cells. Studies in both mice and humans found that nTreg cells constitute around 10% of CD4 T cells in the periphery [15][20]. They express FoxP3 before they are released from the thymus, and the expression of TGF-β helps in their maintenance of inhibitory function after they migrate from the thymus [3][14][3,19]. Inducible Treg cells develop from naive T cells in the secondary lymphoid organ upon antigen exposure. Following interaction with TCR, TGF-β induce the FoxP3 expression in CD4+ CD25− cells, thereby, converting them to FoxP3+ CD4+ CD25+ cells [16][21]. These iTreg cells mediate their inhibitory activities by secretion of IL-10 or TGF-β, which is crucial for inhibiting overexuberant immune response [17][22] (Figure 1).
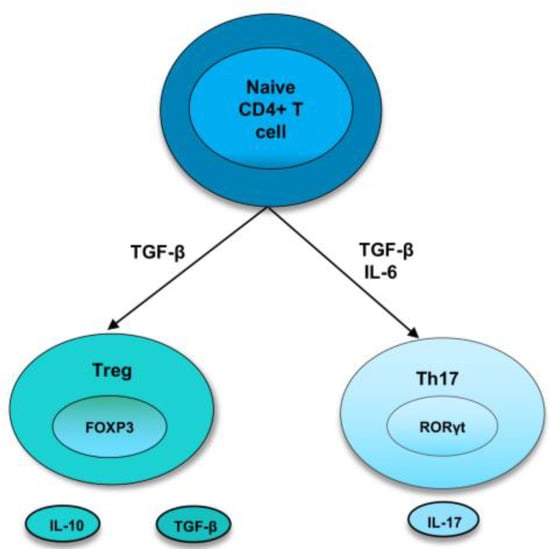
Figure 1. Differentiation of naive T cells into Th17 and Treg cells. In naive CD4+ T cells, TGF-β induce the development of Tregs by promoting Foxp3 expression. Treg cells express cytokines, IL-10 and TGF-β. However, in the presence of IL-6 and TGFβ, RORγt is induced, leading to a Th17 phenotype.
2. Th17/Treg Cells in Lung Inflammatory Diseases
2.1. Chronic Obstructive Pulmonary Disease (COPD)
COPD is a chronic inflammatory lung disease characterized by airway and/or alveolar abnormalities that cause obstructed airflow from the lungs [18][23]. Studies over the last decade highlighted the relevance of maintaining the balance between Th17 cells and Treg cells to control the inflammatory response in COPD. An increased Th17 response is involved in the progression of Chronic Obstructive Pulmonary Disease (COPD) in both clinical and experimental studies [18][23]. Th17 cytokine, IL-17A, levels were higher in the sputum of patients with COPD stages 3 and 4 compared to non-smokers and healthy smokers [19][24]. Reduced numbers of Treg cells were observed in the bronchial epithelium of severe/very severe COPD patients than in those with mild and moderate COPD and healthy smokers [20][25].
