1. Pressurized Liquid Extraction (PLE)
1.1. General Aspects of PLE
PLE is an extraction technique that consists of the removal of analytes present in a solid matrix by applying high temperatures (
Textr) and pressure (
Pextr), usually up to 200 °C and over 200 bar, respectively according to Nieto et al.
[1][22], without reaching the critical point using liquid solvents
[2][23]. These conditions increase solubility and mass transfer rates, resulting in increased solvent diffusivity and, as a result, improved matrix kinetics
[3][17].
Temperature, pressure, time, number of cycles, sample weight and solvent all influence extraction yield. To improve the efficacy of PLE, these parameters should be optimized by using a proper experimental design
[1][22].
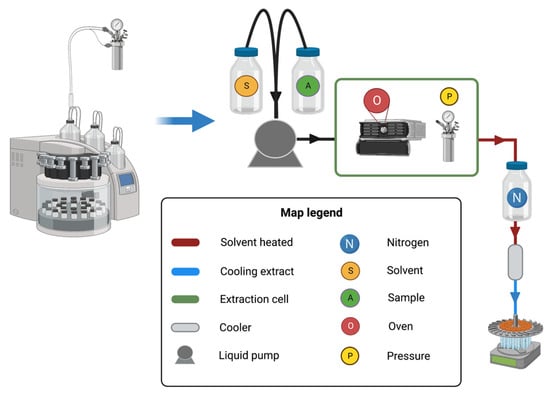
Figure 1 depicts a schematic representation of the PLE extraction equipment’s operation. A high-pressure pump feeds the solvent into an extraction cell and the
Pextr in the system is kept constant
[4][5][24,60]. Because operational
Textr and
Pextr control is critical in this method, the extraction cell is kept in an oven with different valves and restrictors
[5][60]. Moreover, an extract cooler system, a back pressure regulator and a vial to collect the extract are required
[4][24]. Finally, it is important to keep in mind that the equipment must be resistant to corrosion and high pressure
[4][24].
Figure 1. Pressurized liquid extraction equipment and schematic representation of its operation. First, the solvent (S) and the sample (A) are injected into the extraction cell. The extraction cell is composed of an oven (O) and a pressure valve (P) which together allow the achievement of the temperature and pressure selected to extract the compound present in the sample. Then, the extracted compound is cooled and collected in a carousel.
PLE can be used in three modes of action: static mode, dynamic mode and a combination of the two. The static mode is characterized by the use of constant temperature and pressure values, resulting in the sample being in contact with the solvent for a set period. On the contrary, in the dynamic mode, the solvent (usually water) flows continuously through the sample. As a result of the higher volume of the extract obtained, the analytes are diluted in the liquid extract. Analytes are typically pre-concentrated by liquid–liquid extraction or by solid-phase extraction to address this issue. Finally, a combination of both modes of action can be used, which may improve analyte extraction
[2][23].
1.2. Sample Pre-Treatments
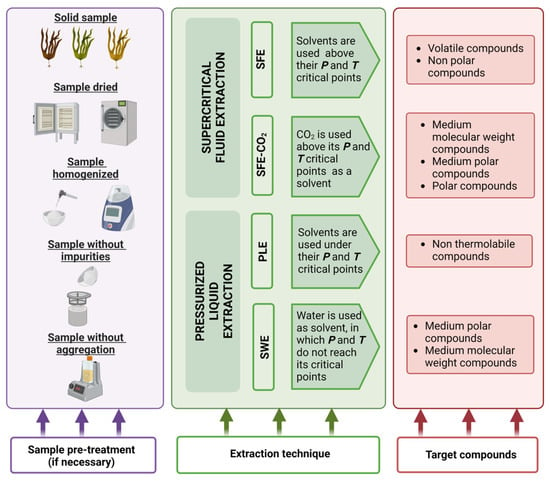
Before using PLE, samples must be pre-treated to increase the contact surface between the solvent and the matrix during the extraction
[2][23]. Pre-treatment can be compiled into four steps, as explained below and in
Figure 2:
Figure 2. Pre-treatment steps, extraction techniques and target compounds from seaweeds using PLE and SWE. In purple, a schematic representation of the steps that should be followed to prepare the sample before the extraction technique is applied. In green, PLE and SPE operational conditions considering water and CO2 as solvents, respectively. In red, a comparison between the compounds extracted using each extraction technique.
- −
-
Drying: the objective of this step is to remove water from the matrix, increasing extraction efficiency
[2][23]. Air-drying, oven heating or lyophilization can all be used, with the latter being the most advantageous because it takes less time and does not degrade the compounds. Indeed, when non-polar solvents are used, this step is critical and a desiccant is commonly included in the PLE cell
[1][22].
-
- −
-
Homogenization: by grinding, the sample should be distributed in a homogeneous manner
[1][22].
-
- −
-
Sieving: this step increases the surface area of the analyte as well as the diffusion of the analyte from the matrix to the solvent
[2][23]. This step yielded a similar particle size in which 2 mm is commonly used for PLE
[1][22]. After sieving you can carry out grinding of the separated portion with greater particle size.
-
- −
-
Dispersion with an inert material: this step is recommended for some samples to avoid aggregation of particles that may lead to alteration in the extraction efficiency
[2][23].
-
1.3. Relevant Parameters in PLE
1.3.1. Solvent
One of the most important parameters to optimize is the extraction solvent
[1][22]. The solvent’s function is to solubilize the target analytes while minimizing the extraction of other components
[2][23]. Therefore, it is important to choose a solvent that has the same polar behavior of the target analytes
[1][22]. Non-polar and water immiscible solvents or a combination of non-polar with medium-polarity solvents are used for non-polar or lipophilic compound extraction. Consequently, solvents with high polarity are used to extract polar and hydrophilic compounds. Finally, when extracting analytes with different polarities, a mixture of solvents with high and low polarity is commonly used. Indeed, some authors suggest following two PLE extractions when the target is for high and low polar analytes, so they can be removed in two steps
[2][23]. As a result, because affinity and miscibility are the two parameters used in predictive approaches to determine the solubility of the target compound in green solvents at different temperatures, experimental trials may be limited
[4][24].
Regarding the application of PLE on seaweeds, using water as a solvent is the most common green technique applied for the carbohydrates extraction since they are more soluble in water at 100–150 °C and the dielectric constant of water is reduced at this temperature. Moreover, subcritical water acts as an acid or an alkali, helping the polysaccharides extraction
[6][47]. A study conducted in 2022 in which PLE was optimized by varying different parameters, showed that temperature was the most critical for the extraction of carbohydrates in microalgae, which can be extrapolated to seaweeds. Furthermore, bioactive polysaccharides extracted from seaweeds using PLE with water are not degraded because temperatures are kept below 200 °C, avoiding caramelization and other degradation reactions
[6][47]. Although water is a good green solvent, it is important to consider that its use may result in unwanted reactions or interference coextraction, affecting the procedure’s selectivity
[3][17]. Therefore, other eco-friendly solvent alternatives, such as deep eutectic solvents, are being considered for carbohydrate extraction. Deep eutectic solvents (DES) are eutectic mixtures composed of hydrogen bonding acceptors (HBAs) and hydrogen bonding donors (HBDs)
[7][61]. Due to their stability, cost-competitiveness, and ease of synthesis, DES have been proposed to dissolve different polysaccharides such as cellulose, starch, chitin and lignin for biomass processing. Moreover, DES was recently used as a solvent to extract fucoidans and alginates from brown algae.
When selecting a solvent, it is also important to consider subsequent steps of the process, such as the clean-up step or concentration step if necessary. Selectivity is the parameter that determines whether or not purification and concentration are required, and it is critical when developing a green technique process
[8][69]. Finally, the solvent used must be both physically and chemically stable. Water, ethanol, organic esters such as ethyl acetate and ethyl lactate, (+)-limonene and their mixtures are the most commonly used solvents in PLE
[4][24].
In 2017, for example, a study on the accuracy of some green solvents with PLE for the fucoxanthin extraction was conducted. Limonene, ethyl lactate and ethyl acetate were selected as green solvents and their ability to extract fucoxanthin was compared to that of ethanol
[8][9][69,70]. The highest yields were obtained for each solvent when the operating temperature was set to 100 °C. None of the green solvents reached ethanol’s yield, with ethyl lactate had the highest percentage. Despite the yield results, limonene had the highest selectivity (expressed as the ratio of total carotenoids to total chlorophylls), proving that limonene is a good alternative green solvent for fucoxanthin extraction.
1.3.2. Temperature and Pressure
As previously stated,
Textr and
Pextr are two important parameters to be optimized when using PLE. Elevated temperatures are used to reduce the viscosity of the liquid solvent used, allowing it to a better wet the matrix, and solubilize the analytes of interest. In addition, diffusion of analytes in the matrix surface is facilitated because high temperatures aid in the breakdown of the analyte–matrix bonds
[2][23].
Textr varies between 50 and 200 °C and is dependent on the target analyte. Thus, lower
Textr are selected for extraction of certain bioactive compounds due to their thermolability. Because high temperatures above the atmospheric boiling point are required in order to keep the solvent liquid, a high operational
Pextr is required
[2][23]. Furthermore, high pressure increases the extraction yield by forcing the solvent to enter the matrix pores
[5][60]. In PLE methodology,
Pextr usually varies from 5.0 to 15 MPa. These high-pressure and temperature conditions allow for the extraction of the target analytes in a short period of time while using less solvent and showing a recovering ability in terms of extraction yield similar to other techniques
[1][22].
1.3.3. Time and Number of Cycles
The time of extraction is defined as the duration of direct solvent contact with the sample for a given
Textr and
Pextr [4][24]. This value is determined by a variety of factors, including the mode of action. When using static mode d, extraction time is reduced (
textr = 5–20 min)
[4][24]. On the contrary, when the dynamic mode is established, the flow of the solvent must be determined to select
textr. Furthermore, it is significant to notice that low flows cause PLE system blockages while high flows result in diluted extracts. Finally, it is known that several cycles with low volume lead to higher yields of the target analyte, while a single extraction with a large amount of solvent does not correspond with higher extraction yields
[4][24].
1.4. Post-Extraction Treatment (Clean-Up)
During PLE, some compounds of the matrix could be co-extracted causing interferences, so a clean-up step could be necessary to decrease the limit of detection (LOD) value
[1][22]. Extraction and clean-up steps can be carried out simultaneously, which leads to a reduction in time and quantity of solvents used, between 15% and 52%
[10][78]. Different clean-up techniques can be used:
- −
-
On-cell clean-up technique: the solvent is passed through the cell to elute interferences prior to the extraction step. This reduces the extraction time required and enables the process to be automated. On the other hand, the analyte must have a different polarity than the compounds that cause the interference. Moreover, finding a solvent capable of eliminating the interferences without causing damage to the analytes can be difficult
[10][78].
-
- −
-
Liquid chromatography techniques: they are used to remove interferences from complex matrices. The most used techniques are normal phase liquid extraction (NPLE) and gel permeation chromatography (GPC). On the one hand, NPLE is used as a preparative chromatography in a glass column filled in-house with the stationary phase
[10][78], whereas GPC is a purifying technique based in the separation depending on the molecular size of the compounds. The main advantages of GPC are its ability to be automated and the clean-up capacity maintenance for months. Divinylbenzene-linked polystyrene gel is the main material used for GPC
[1][22].
-
2. Combinatorial Approaches of PLE with New Extraction Methodologies
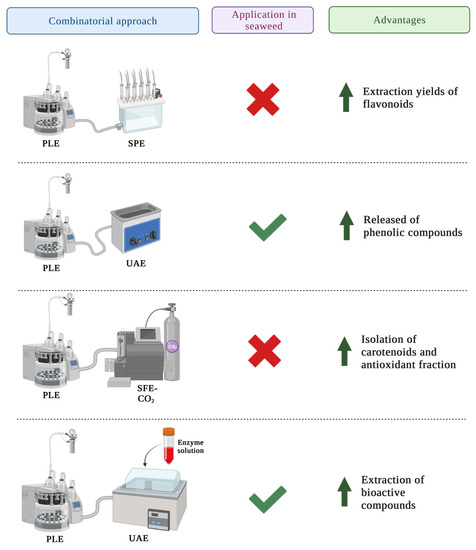
There have been few studies on the use of PLE in seaweeds due to its novelty. However, following the green technology trends, PLE could be combined with other methodologies to improve bioactive compound extraction efficiency and reduce solvent and time consumption. Moreover, because combinatorial approaches of PLE applied in the extraction of bioactive compounds of seaweeds have not been thoroughly studied, results of trials where PLE and other techniques are applied in other matrices are shown in this section and compiled in Figure 3 as case studies for future applications of these techniques combined.
Figure 3. Improvements achieved in the recovery of compounds from different matrices when PLE is combined with other extraction techniques.
2.1. PLE Combined with SPE
The combination of PLE and SPE has been used for the separation of specific phenolic compounds
[11][79]. The mode of action of this combination is based on the ability of PLE to extract bioactive compounds from the matrix and the ability of SPE to purify the extracted compounds
[12][80]. Thus, SPE is mainly used as a post-extraction technique since PLE is a non-selective methodology.
There are no data on the use of PLE and SPE for the extraction of phenolic compounds from seaweeds, but it was applied in other matrices such as apple pomace, mate leaves and lemon peel. Higher yields of total flavonoids were obtained in all the three matrices, when compared to the extraction using PLE alone
[11][12][13][79,80,81]. On the contrary, when lemon peel was used as the matrix, the yields of the polar compounds were lower, while total phenolic acids and flavonoids showed no statistical difference between PLE and PLE combined with SPE in mate leaves
[12][13][80,81].
2.2. PLE Combined with UAE
Some studies have combined the use of PLE with UAE (UAPLE) in different matrices, including seaweeds. A recent study combined PLE and UAE to extract phenolic compounds from three brown and one red algae. The operational conditions (solvent 80% MeOH:H
2O (
v/
v); 10 mL; 130 °C; 130 bar; two static cycles of 10 min) were able to increase the release of phenolic compounds from the matrix due to the high and stable pressure
[14][82]. Nevertheless, further research is needed to know if scale-up is available from the economic point of view
[15][83], since many companies have difficulties because of the high expense with facilities, extraction time and ultrasound power
[16][84].
2.3. PLE Combined with SFE-CO2
The combination of PLE with SFE-CO
2 is a sequential process based on the ability of SFE-CO
2 for the extraction of the lipophilic fraction of the matrix, and the ability of PLE for the extraction of the antioxidant or high polarity compounds
[17][85]. Because there is no information available about this sequential process used in seaweeds, results from other studies were compared to determine if this methodology could be useful for extracting seaweed compounds. For the recovery of bioactive compounds from rowanberry pomace using SPE-CO
2 and PLE consecutively, results showed that this is an effective method for the isolation of carotenoid-rich and antioxidant-rich fractions
[18][86]. Same conclusions were achieved when this sequential process was applied in
Viburnum opulus pomace and berries
[17][85]. Moreover, an economic evaluation of this process applied in passion fruit by-products was carried out in Brazil.
2.4. PLE Combined with EAE
The application of EAE as an extraction technique leads to some disadvantages, such as the high cost of enzymes, the limitation of cell disruption because of the specificity of the enzyme and the inactivation of enzymes with parameters such as temperature and pH change
[19][88]. To solve these limitations, EAE studies combined with other new methodologies have been performed. For example, when EAE was combined with alkaline hydrolysis and PLE for the extraction of bioactive compounds from
Sargassum muticum, the extraction yields were higher than when PLE was used alone
[20][89]. This could be explained by the formation of a protein–polyphenol complex which results in decreased polyphenol recovery. This complex may be formed when the enzyme disrupts the cell of the seaweed, releasing proteins and other compounds. Thus, these compounds may form complexes with polyphenols, resulting in aggregation and precipitation and ultimately, lower yields
[20][89]. Because no additional information was discovered, more research is required to determine whether combining EAE and SPE could result in higher yields.
Given the current data on PLE combined with various new methods, a sequential process using PLE and SFE-CO2 should be considered because extraction yields are increasing, and an industrial scale appears to be feasible. However, because there has been no research on the application of a sequential process involving PLE in seaweeds and different parameters affecting the percentage of recovery, including the matrix, more research is required. Furthermore, because UAPLE produces intriguing results, scale-up trials should be conducted to determine whether this sequential process is economically feasible.
3. Evaluation of Pressurized Liquid Extraction (PLE) Applications
As mentioned in previous sections, using PLE to recover seaweed compounds results in the extraction of various bioactive compounds. Because of their technological function, importance as functional ingredients, or application in innovative food packaging systems, these compounds can be used in the food industry.
3.1. Technological Function and Functional Ingredients
Different compounds extracted from seaweeds are already used in the food industry for the technological improvement of food products, such as carbohydrates from seaweeds which are mainly used for its functional properties. Thus, agar is applied in the confectionary industry for its hydration maintenance capacity. Moreover, the addition of agar in meat products allows the reduction of the fat content in the final product
[21][90]. The use of seaweed extracts in meat emulsions is interesting from a technological point of view since a harder and chewier structure with better water and fat binding is achieved
[22][91]. For example, due to the increase in vegan and vegetarian diets, meat analogues are increasingly in demand and carrageenan is used because of their stability properties
[21][90]: it is already used in low-fat sausages, beef burgers and beef patties as a thickener and stabilizer agent
[23][92]. In addition, since algae have essential micronutrients such as Mg, K and Fe, and low Na content, the addition of these extracts into meat products may be a good opportunity to increase the nutritional value of these products
[24][93]. Carrageenan and alginates have been added in bakery products such as bread, being able to reduce the moisture loss during storage and the dehydration rate of the crumb. Additionally, alginate was able to retard the hardening of the crumb
[25][94]. Fruit jellies, donuts and cakes are also examples of products where agar is added
[21][90]. At last, the use of alginates has an antimicrobial growth activity in the vegetable industry and is a good choice for encapsulation and delivery systems of probiotics, according to the bibliography
[21][90].
On the other hand, proteins, peptides and amino acids are mainly used as stabilizers, thickener agents, protein replacements and gelling agents
[26][95]. Peptides extracted from different seaweeds were incorporated in pasta products, showing superior pasta quality and antioxidant properties over the control
[26][95]. Furthermore, the addition of
Palmaria palmata hydrolysate in bread improved texture and sensory acceptability. In addition, several studies of peptides extracted from seaweeds have been carried out, and Wakame peptide jelly and Nori peptide S are two bioactive peptides included in some Japan foods due to their antihypertension activity
[27][4]. Nutritional supplements are also considered in the scope of the food application of seaweed extracts. As seaweeds are a good source of proteins, not only because of the quantity but for the quality, the use of this extract as supplementation would be a good option for those athletes following a vegan diet
[28][96]. Thus, there are already products on the market, such as Solaray, that contain extracts of
Rhodymenia palmata, which helps in the maintenance of the immune system health
[29][77].
The incorporation of phlorotannin into food formulations may be limited because of their astringency and bitter taste. Thus, these compounds were encapsulated into nanofibers made of sodium alginate and polyethylene oxide and successfully incorporated into chicken breasts. The chicken was stored, and thanks to the phlorotannin’s encapsulation,
Salmonella growth was prevented, while the sensorial characteristics of the product were unaffected
[30][97]. Moreover, the preservation ability against polyphenol oxidase activity and melanosis formation was proved during white shrimps’ ice storage when phlorotannin extracted from
S. tenerimum were added. Furthermore, when shrimps were immersed in 5% phlorotannin solution, shelf life was extended by 4 days and higher scores on overall sensory acceptability when compared to control
[30][97].
Finally, due to the current trend of changing the soy- and animal-derived protein sources for animal feed, seaweed extracts have been also incorporated into these products
[26][95]. According to one study, incorporating algae extracts into dairy cattle feed resulted in higher I and Se content
[31][99]. In fact, it has been demonstrated that including seaweed extracts in animal feed is a good way to achieve the I daily intake for those people with I deficiency, since milk excretion meets the needs of this mineral
[26][95]. The incorporation of red seaweed extracts to poultry feed was also studied. The results show that incorporating
Sarchodiotheca guadichaudii and
Chondrus crispus extracts into layer feed improves the growth of beneficial bacteria and reduces
Clostridium perifringens proliferation in the gut, thus improving the safety of the products obtained. Moreover, the egg yolk and weight were increased by adding 1% of
Sarchodiotheca guadichaudii into the feed without altering the color of the yolk and the shell thickness
[32][100]. Extracts from seaweeds are also being studied for feeding fish, especially protein extracts, since they are the most expensive dietary requirement for fish and shellfish aquaculture. Moreover, seaweed is also a good source of highly unsaturated fatty acids. Considering the requirements for fish nutrition, studies suggest that seaweed extracts may be a good option for fish feed, allowing the substitution of animal meal by plant meal in these products
[26][33][95,101].
Considering the advantages of using PLE alone or in combination with other green techniques to obtain previously exposed seaweed bioactive compounds, and how food products may improve with the addition of these biomolecules, the incorporation of seaweed extracts may be a good strategy to enhance their nutritional profile and the technological properties.
3.2. Application as Innovative Food Packaging
Some compounds extracted from seaweeds such as laminarin, phlorotannin, flavonoids, terpenes, lactones and proteins are active against bacteria and fungi cells and bacteria biofilm formation (which is more difficult to remove)
[34][116]. The correct preservation of organoleptic characteristics, while avoiding microbial growth during the storage as well as the need to extend the shelf life of the products, are critical for the food industry because these are factors deeply involved with the increase in food waste. Therefore, the use of antimicrobial compounds extracted from seaweeds may be a good option for increasing the shelf life of food products.
Sensory analyses of different products with some of these antimicrobial compounds were carried out to identify their impact on different parameters such as flavor, taste, color and smell. The results showed that edible film made of chitosan and seaweed extracts from
H. longata and
P. palmata inhibit the growth of mesophilic and psychrophilic microorganisms by maintaining the pH and water activity without affecting the sensorial characteristics of fish burgers. On the contrary, the sensory evaluation of pork patties with fucoidan and laminarian extract in a ratio of 0.5
w/
w proved an adverse impact on the product. To avoid a possible negative impact on the organoleptic characteristics of the products, adding the antimicrobial compounds onto the packaging instead of adding them directly in the product could be an option. In fact, considering that most of the spoilage and contamination of food occurs on its surface, adding these compounds in the packaging may extend the shelf life of the product without affecting its organoleptic characteristics
[34][116].
In terms of packaging, seaweed derivatives were studied to determine their suitability for bioplastic production. Bioplastics are synthetic plastics derived from biodegradable sources and their main disadvantage is their hygroscopicity
[35][117], which affects the mechanical and storage properties required for food packaging. Furthermore, edible coatings are thin membranes composed of GRAS such as polysaccharides, lipids and proteins. Moreover, edible coatings can act as carriers of different bioactive compounds useful in food preservation. Thus, these special coatings maintain firmness, inhibit microbial growth and prevent food weight loss during long-term storage
[36][50]. Carrageenan is a polysaccharide that can be used as an additive combined with other compounds for bioplastic production since it has low water vapor permeability (WVP)
[35][117]. A biodegradable film made of olive extract, glycerol and 1% of carrageenan (
w/
v) showed good mechanical and antimicrobial properties. Moreover, a bionanocomposite made of 10% of gelatin (
w/
v), 0.5% (
w/
v) of k-carrageenan and 1, 3 and 5% of nano-SiO
2 showed a drop in the WVP from 100% to 68%. Finally, the bioplastic production using starch, glycerol and 5% of carrageenan showed that the addition of carrageenan enhanced the moisture resistance, brittleness and the tensile properties of the polymer. Thus, carrageenan can be used to produce edible food packaging
[35][117].
Alginates are another type of polysaccharides that could be used in the formulation of biodegradable films because their main properties are tensile strength, elongation and WVP that are suitable for biodegradable packaging
[35][117]. To determine how mechanical properties were affected, alginate biofilms were compared using hydrophilic and hydrophobic plasticizers. Tributyl citrate (TC) showed better results because TC and alginate secondary interactions improved mechanical resistance. Furthermore, it was proved that elongation at break can be increased by using hydrophilic plasticizers such as glycerol
[35][117]. A study using alginate with aloe vera (AV) and garlic oil (GO) in different proportions to produce a brand-new edible coating was performed to determine their UV shielding, thermal and antimicrobial properties. The results showed that after 16 days of storage, tomatoes with the edible coating made of 33.3% alginate, 66.7% AV and 5% GO showed an 8% mass loss while tomatoes without edible coating showed a 47% of mass loss. In addition, tomatoes with 33.3% alginate, 66.7% AV and 5% GO as edible coating suffered less damaged in the UV light measurement and showed better elongation break properties. For the inhibition growth of
Staphylococcus aureus,
Escherichia coli and
Syncephalastrum racemosum, better results were obtained when tomatoes were coated with 33.3% alginate, 66.7% AV and 5% GO. On the contrary, better tensile strength results were obtained in tomatoes with 33.3% alginate and 67.3% AV
[36][50].
Based on the data presented, the incorporation of alginates, carrageenan, laminarin, phlorotannin, flavonoids, terpenes and proteins onto innovative food packaging is a viable option for this innovative pathway.