Microaneurysms (MAs), a characteristic feature in diabetic retinopathy (DR) and diabetic macular edema (DME) DR and DME, can be detected by fluorescein angiography, optical coherence tomography (OCT) and OCT angiography. These instrumental analyses demonstrated a geographic and functional association between MA and ischemic areas. MA turnover, the production and loss of MA, reflects the activity of DME and DR. Several cytokines are involved in the pathogenesis of MAs, which is characterized by pericyte loss and endothelial cell proliferation in a vascular endothelial growth factor (VEGF)VEGF-dependent or -independent manner. Ischemia and MAs localized in the deep retinal layers are characteristic of refractory DME cases. Even in the current anti-VEGF era, laser photocoagulation targeting MAs in the focal residual edema is still an effective therapeutic tool, but it is necessary to be creative in accurately identifying the location of MAs and performing highly precise and minimally invasive coagulation. MAs play a distinctive and important role in the pathogenesis of the onset, progression of DR and DME, and response to anti-VEGF treatment. Further research on MA is significant not only for understanding the pathogenesis of DME but also for improving the effectiveness of treatment.
- diabetic retinopathy
- diabetic macular edema
- microaneurysms
1. Introduction
2. Clinical Feature of Microaneurysms
2.1. Diabetic Retinopathy (DR)
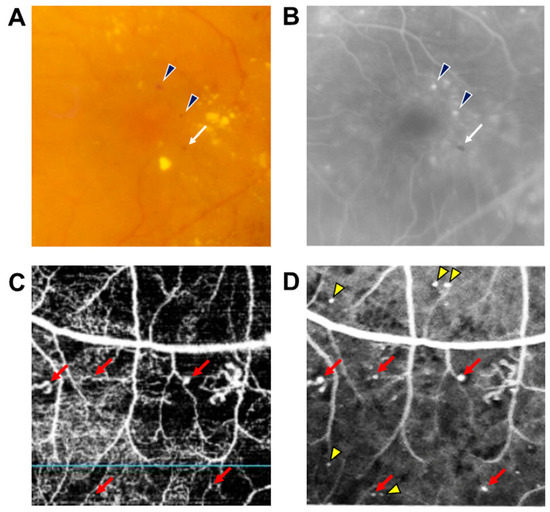
DR results from changes in retinal microvascular structures and blood flow due to persistent hyperglycemia [4]. MAs are usually the earliest manifestations of DR and appear as tiny red dots scattered throughout the retina as well as the hallmark of clinical diagnosis of DR. If the MAs that leak were not present in the macular area, MAs do not have any manifestations and do not affect the vision of patients; however, early recognition of MAs can lead to early detection and treatment of DR that will reduce the likelihood of vision loss. MAs cannot be distinguished from tiny dot hemorrhages, and they can be occasionally undetectable ophthalmoscopically. For this reason, fluorescein angiography (FA) has been considered the gold standard for the detection of MAs, visualized as hyperfluorescent dots on early phase, while the dot hemorrhages are visualized as hypofluorescent dots (Figure 1) [5].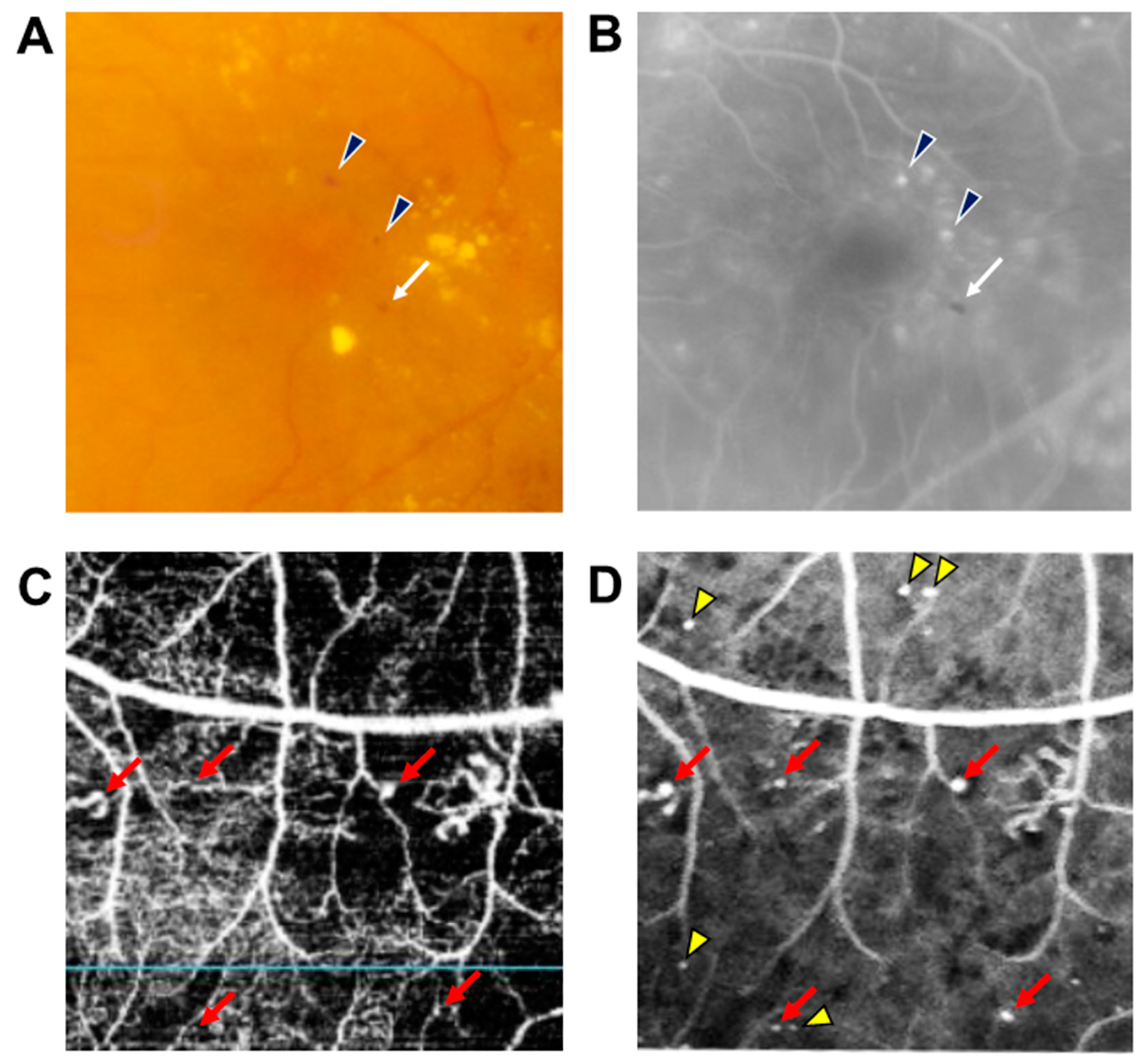
2.2. DME
2.3. Distribution Pattern of MAs in Diffuse DME
Using ultra-widefield FA, a study showed that the larger the area of non-perfused retina and the greater the severity of DR, the more likely it is to be diffuse DME; conversely, the smaller the level of ischemia, the greater the possibility of focal DME [10][17]. This finding suggests that the type of DME progresses from focal to diffuse as retinal ischemia worsens. MAs are usually seen in the border areas of CDOs in DR [11][18]; this association results in a characteristic distribution of MAs in diffuse DME (Figure 2) [12][19]. There were more MAs in the periphery than in the central area of the edema. Although CDOs in the periphery of the edema are small, they have a large circumference and have fine irregularities and fragmentation. Furthermore, since there are more CDOs in the periphery of the edema, there are also more MAs adjacent to them as compared to CDOs in the center of the edema [12][19]. Active leakage from several MAs in the edema periphery would contribute to the expansion of edematous areas. In fact, severe ischemia leads to large size edema that extends beyond the macular area [13][20].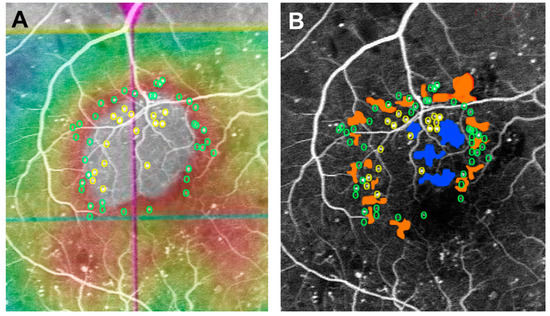
3. Pathology of MAs in DR and DME
3.1. VEGF May Potentially Induce the Development of MAs
3.2. Mechanisms of Pericyte Dropout
The theory that VEGF produced by ischemia induces MA formation does not explain why MAs are the first morphological abnormality in DR as there is also a VEGF-independent mechanism for MA formation. MAs are accompanied by endothelial hyperplasia resulting from aberrant proliferation, basement membrane thickening, and a decreased number of pericytes [2][20][2,35]. Pericyte loss occurs in both diabetic and galactose-fed dogs and is characterized by changes in retinal vessels, such as MAs, hemorrhage, and the formation of non-perfused areas, similar to those seen in human DR [21][22][23][36,37,38]. Experimental evidence suggests that these changes can be prevented by aldose reductase inhibitors (ARI) [21][24][25][36,39,40]. Apoptosis was not observed in galactose-exposed retinal ECs that have low AR content and activity. On the other hand, AR-overexpressing ECs showed decreased cell viability and polyol accumulation, similar to that in pericytes. This suggests that the physiological difference in response to hyperglycemia is attributable to the level of AR expression and is not a cell-specific feature of pericytes and ECs. A study employing a co-culture system of pericytes and ECs exposed to a high-glucose medium demonstrated that there was an increased proliferation of ECs as the number of pericytes decreased. Biochemical assays disclosed that the levels of active transforming growth factor-beta (TGF-β) in media were linked to EC growth. Supplying active TGF-β to a co-culture medium containing high-glucose restored the inhibitory activity against EC growth.4. Clinical Role of MAs in the Management of DR and DME
4.1. MA Turnover Is a Biomarker for Disease Activity and Treatment
MAs do not remain stable in the retina in DR and DME for long periods of time. The appearance and disappearance of MAs, defined as MA turnover, represent a dynamic process and reflects disease activity, and it can be a predictor of DR and DME progression. A 5-year prospective longitudinal study demonstrated that MA turnover and MA formation rates are related to the development of vision-threatening complications, such as DME and proliferative DR, and the worsening of DR [26][44].4.2. MAs Is Associated with Resistance to Anti-VEGF Therapy
In DME treatment, anti-VEGF therapy is effective for reducing the retinal thickness and decreasing the size of edematous areas; however, residual focal edema frequently remains, as seen in 65.8% of cases after the first injection [27][48]. An analysis using a 3D mode OCT map wherein an edematous area was divided into 100 sections showed that the reduction in retinal thickness after anti-VEGF therapy varied in regions of the DME [28][49]. A 10–20% reduction in retinal thickness accounted for approximately 40% of the total edematous areas, whereas only 6.4% of the edematous areas showed a reduction in retinal thickness of 30% or more. Areas with a reduction in retinal thickness of less than 5% were indicative of refractoriness to anti-VEGF therapy, and they accounted for approximately 10% of the edematous areas. These results suggest that the edema-improving effect of anti-VEGF therapy varies by site and that some sites are less responsive than others.4.3. Direct Photocoagulation Aiming MAs
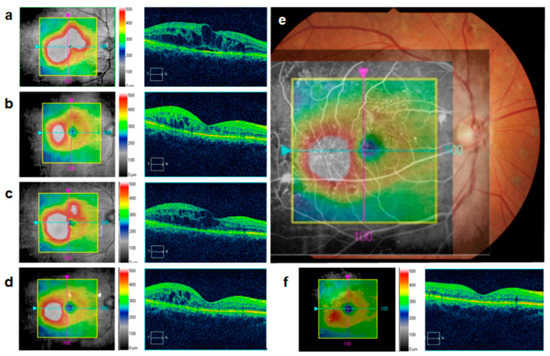
After anti-VEGF injection into the areas involved in DME, the appearance of the fovea usually returns to almost normal; however, focal edema often persists in the paracentral area. If injections are discontinued because edema has improved in the central area and results in improved visual acuity, the residual perifoveal edema may expand and affect the central areas, as shown by the sample case in Figure 34. Hence, MAs within the residual edema should be targets of additional treatment.