Nucleation is a key process for the growth of diamond films. Spontaneously nucleation on heterogeneous substrates is difficult. This is mainly because the high surface energy of diamond. Rapid nucleation (a few minutes commonly) is a necessary condition for the deposition of high-quality diamond films. The characteristics of the substrate, such as surface defects, surface energy, surface diffusion and bulk diffusion of atoms, and chemical reactivity, affect the diamond nucleation process. Especially, a gallium nitride (GaN) substrate, which has a large lattice mismatch and thermal expansion mismatch with diamond, puts forward some difficult requirements for diamond nucleation. The temperature of the substrate also affects the diamond nucleation process. Considering the quality and rate of diamond nucleation and the thermal stability of GaN high electron mobility transistors (HEMTs), researchers regard ~600 °C as a more suitable nucleation temperature.
1. Nucleation through Ultrasonic Particle Treatment
Ultrasonic pretreatment in a diamond powder suspension is performed to form scratches on the substrate surface or attach diamond particles to the substrate as nucleation sites
[1][59]. The liquid used for the suspension is mainly ethanol, acetone, and pure water, etc.
[2][60]. This method is relatively simple to operate, because there is no excessive requirement for the substrate’s geometry, and no special deposition equipment is required. In the experiment by Mohapatra et al.
[3][61], to enhance the density of diamond nucleation sites, the substrate was ultrasonically treated in a 2 μm-sized diamond powder suspension for 10 min, with subsequent cleaning with acetone and deionized water in an ultrasonic bath. Continuous diamond films with a high nucleation density were obtained. However, the core purpose of the ultrasonic pretreatment method is to damage the substrate surface and create residual diamond particles to increase the nucleation density, which makes the substrate damaged or roughened
[4][56]. Therefore, it is limited to use in the subsequent experimental study of diamond film growth on GaN. Especially, for the technical method of directly depositing diamond on the top of GaN HEMTs, this pretreatment process is likely to cause damage to the AlGaN barrier layer and affect the further use of the devices. This method is not very suitable for this growth technique approach.
2. Bias Enhanced Nucleation
The use of a biased voltage in a CVD chamber to promote diamond nucleation is called Bias Enhanced Nucleation (BEN), which was first proposed by Yugo et al.
[5][62]. Lifshitz et al. detailed described the bias-enhanced nucleation process and proposed the corresponding mechanism
[6][63]. As mentioned above, Oba and Sugino have used bias enhanced nucleation prior to diamond deposition onto GaN materials in a microwave plasma chemical vapor deposition chamber
[7][64]. However, they could not grow a continuous film due to the low nucleation density. In 2011, Alomari et al. reported a 0.5 μm-thick NCD film process on In
0.17Al
0.83N/GaN HEMTs via hot filament chemical vapor deposition at 750–800 °C
[8][65]. After NCD deposition, the
fT and
fmax of the device were 4.2 and 5 GHz, respectively. Moreover, the DC characteristics were basically similar to those before deposition. However, the RF-tested device showed high gate leakage, probably due to the effect of the high-temperature and hydrogen-rich deposition environment on the gate dielectric and gate metal. In 2014, the NCD layer was increased to a 2.8 μm thickness
[9][66].
BEN can greatly improve the nucleation site density before diamond growth, providing a prerequisite for the high-quality nanocrystalline diamond. The typical nucleation density achieved by BEN is in the order of 10
10 cm
−2. However, the BEN method also has some disadvantages, such as being only applicable to conductive substrates
[4][56].
3. Electrostatic Seeding
At present, electrostatic seeding is the most widely used pretreatment method for enhancing the nucleation of polycrystalline diamond films
[10][67]. The main process is to establish a heterogeneous charge difference between the substrate and the diamond powders and use electrostatic adsorption to realize the diamond powder seeding
[11][12][68,69]. Specifically, by cleaning the substrate or directly coating a suitable polymer layer on the substrate surface, the nanodiamond powders with opposite potential are self-assembled on the substrate to form a uniform single-particle diamond powder layer.
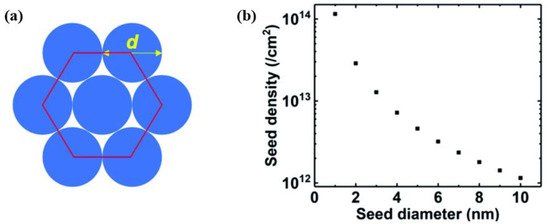
Figure 14 shows a schematic diagram of perfectly spherical seeds densely packed in hexagons and the theoretical maximum seeding density with different seed diameters. The preparation of a stable and uniform diamond colloidal suspension is one of the important prerequisites. Diamond is insoluble in water or any other solvent, which helps the corresponding diamond colloidal suspension form. The colloidal suspension stability is determined by the suspended particles’ potential, and it is generally believed that an absolute value of the ζ-potential more than 30 mV will create a good condition for forming a stable colloidal suspension
[13][52]. It should be noted that the amount of nanodiamond particles added to the solvent is also the critical factor. Too few nanodiamond particles may not meet the high-density diamond particle seeding requirement. Too many nanodiamond particles will lead to a great increase in the collision probability between particles. This process will make the van der Waals forces between particles possible to overcome the repulsive potential caused by the surface charge and finally lead to agglomeration. In addition, the diamond particle size will also have a certain impact on the colloid stability
[14][70]. Obtaining a uniform monodisperse colloidal suspension is an essential part; the colloid is conducive to improving the seeding quality and increase seeding density
[12][15][16][69,71,72]. Smaller particles currently used for seeding treatment are produced via the detonation of unused or decommissioned explosives, and these diamond particles are called detonation nanodiamond (DND). In the process of producing nanodiamond via detonation, a large amount of sp
2-phase carbon is formed, and it is precisely because this part of non-diamond carbon promotes the formation of large diamond particle agglomerates. The existence of agglomerates makes it difficult to obtain monodisperse diamond colloids. The methods of preparing monodisperse single nanodiamond particulates are mainly high-power ultrasonic treatment, stirred-media-milling method, and dispersant assisted dispersion. An in-depth discussion about preparing monodisperse nanodiamond colloids is beyond the scope of this re
svie
archw.
Figure 14.
(
a
) Arrangement model of spherical seeds in hexagonal close packed structure. (
b) Maximum possible seed density based on corresponding model [4]. ) Maximum possible seed density based on corresponding model [56].
In 2007, Ozawa et al. obtained the first DND (4–5 nm sized particles) monodisperse colloidal suspension via a wet milling process using zirconia beads to help disperse diamond powders
[17][73]. Due to the smaller gap between particles, the smaller diamond particles can effectively achieve a high seed density. Stehlik et al. used 2 nm DND as diamond seeds to obtain a seed density of up to 2.88 × 10
13 cm
−2 [18][74]. As mentioned above, the reason why a high seeding density can be formed on the substrate surface is that there is a certain potential difference between the substrate surface and the DND colloid. Changing the condition, such as the pH value and surface potential, can control this part of the potential difference.
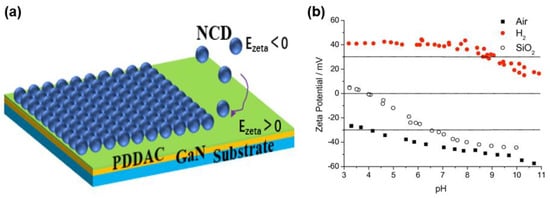
Figure 25 shows the ζ potential of a hydrogenated and oxidized diamond colloid solution measured over a wide range of pH values. Mandal et al. studied the potential changes in the Ga-face and N-face GaN with pH in detail
[19][75]. Between pH 5.5 and 9, both showed a negative ζ potential, while the potential of N-face GaN showed a more negative ζ potential, which was mainly due to the larger concentration of adsorbed oxygen on the surface. Some researchers have also tried to change the surface potential ζ by covering a polymer layer on the substrate
[20][21][22][23][76,77,78,79]. This polymer will undergo corrosion decomposition during the CVD heating process, leaving nanocrystalline diamond seeds on the substrate surface. Currently, the polymers commonly used are poly-diallyldimethylammonium chloride polymer (PDDAC), polystyrene sulfonic acid (PSS), and polyvinyl alcohol (PVA) etc., and the process adsorbed on the surface of the substrate particles or polymer mass is determined by the Sauerbrey equation. At present, this technology has been able to achieve selective seeding on the substrate and allow ND deposition matching complex 3D structures
[11][20][22][24][25][58,68,76,78,80].
Figure 25. (
a) The electrostatic grafting of negatively charged diamond nanoparticles on a cationic polymer-coated substrate. (
b) Zeta-potential of a hydrogenated (H
2) and oxygenated (air) diamond colloid measured over a wide range of pH values
[12][69].
In addition, nucleation is also affected by process parameters. Too high pressure in the reaction chamber will lead to a decrease in the nucleation rate. An excessive substrate temperature and carbon concentration will make the quality of diamond films decrease. Otherwise, it will affect the uniformity of nucleation. Moreover, the material of the substrate will also have a significant impact on the nucleation process. This section aims to focus on the effect of pretreatment methods on nucleation, and the dependence on the temperature is slightly mentioned. Temperature is generally a key parameter of the nucleation by increasing the surface diffusion of atomic species. Many examples of enhanced nucleation with temperature were found with different deposition methods, and an optimum temperature has been found to exist at ~860 °C where the nucleation density reaches a maximum. Reactive species or molecules are physically (<900 °C) and chemically (>900 °C) adsorbed on the substrate surface. This leads to an abrupt change in the diffusion length at ~900 °C. When the temperature is close to 860 °C, the adhesion probability of the active species on the substrate surface increases
[26][81]. The effect of substrate temperature on diamond nucleation can be obtained from this literature in detail
[27][28][29][82,83,84].