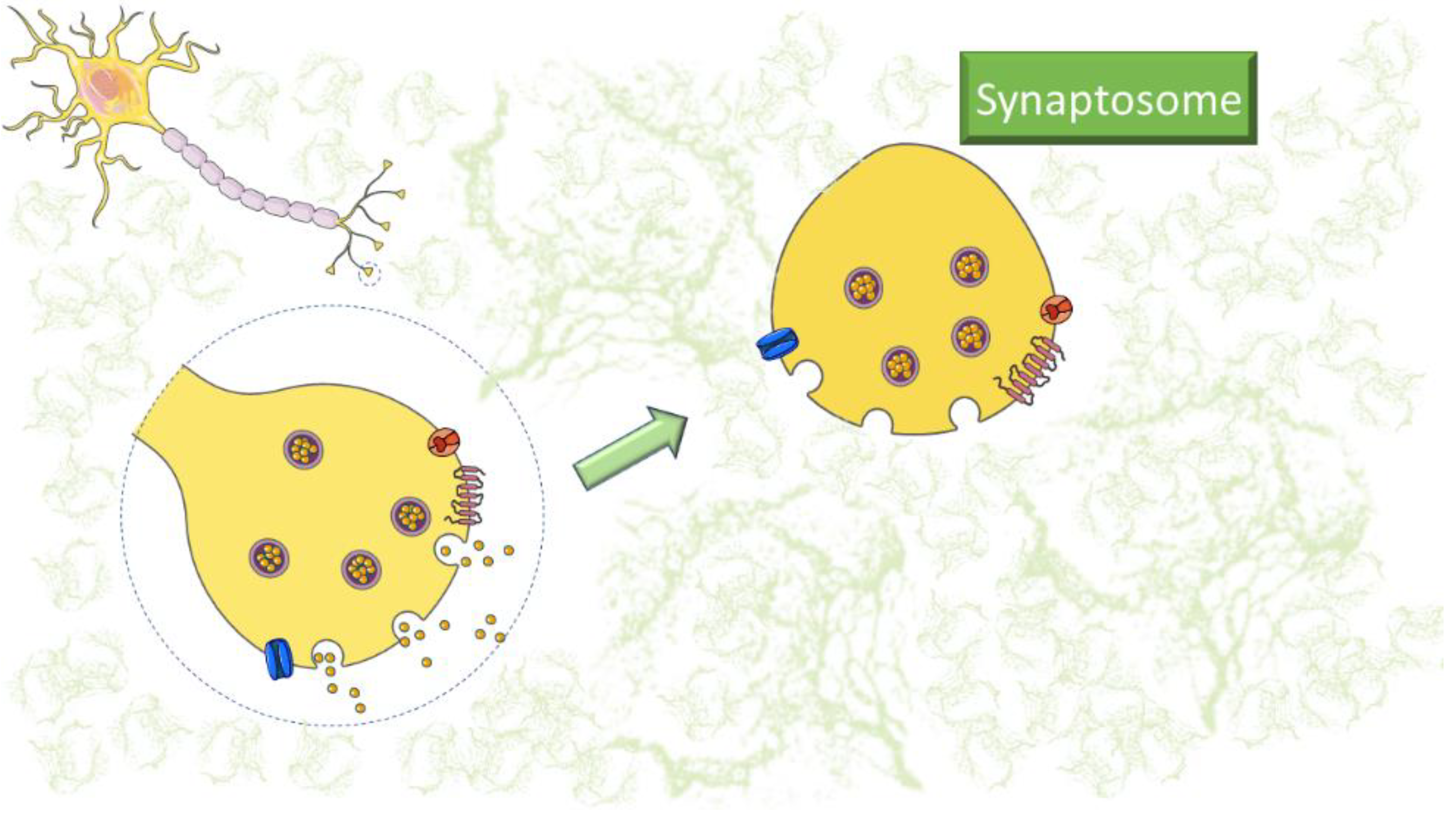
Synaptosomes are subcellular components isolated from nerve terminations that can be prepared by homogenizing brain tissue in isotonic sucrose solution followed by appropriate centrifugation. Their preparation technique has a long history since synaptosomes were first isolated from nerve endings and described by Gray and Whittaker in 1962. The preparation of synaptosomes produces presynaptic boutons alone or in combination with fragments of postsynaptic membranes. Interestingly, synaptosomes contain organelles and vesicles that express native channels, receptors, and transporters. At 37 °C, these isolated nerve endings are metabolically active and synthesize and release neurotransmitters. They are actively used to investigate neurotransmission, its actors, and the mechanisms of neurotransmitter release. To date, many functional and non-functional applications of synaptosomes have been documented. Due to their versatility, synaptosomes have been actively used to study neuroinflammatory processes.
- synaptosomes
- disease
- neuroinflammation
- cytokines
- immune system
| Analytical Technique | Disease | Neuroinflammation Markers | References |
|---|---|---|---|
| Western Blot Flow Cytometry Neurotransmitter release Proteomics Confocal microscopy |
Alzheimer’s disease | ROS | [1,5,11,21,29,32,42,43,44,45,46,47,48,49,50,51,52,53,54][1][5][11][21][29][32][42][43][44][45][46][47][48][49][50][51][52][53][54] |
| C1q | |||
| iNOS | |||
| TNF-α | |||
| IL-1β, IL-6, IL-1 | |||
| CCL2, CCL5, CXCL1 | |||
| Parkinson’s disease | TNFα | [1,26,49,55,56][1][26][49][55][56] | |
| IL-6 | |||
| NOS2 | |||
| ROS | |||
| Huntington’s disease | TNF-α | [1,30,44,55][1][30][44][55] | |
| IL-1β | |||
| IL-10 | |||
| NO | |||
| Epilepsy | ROS | [57,58][57][58] | |
| iNOS | |||
| NMDA-induced excitotoxicity | IL-1β, IL-6, TNFα, mPGES-1, COX-2 | [55,57][55][57] | |
| iNOS | |||
| SAE | IFN-γ | [59,60][59][60] | |
| IL-1β | |||
| IL-10 | |||
| TNF-α | |||
| IL-6 | |||
| CXCL1 | |||
| Influenza A Virus | CD80 | [61] | |
| CD36, CD68, | |||
| C1QA, C3 | |||
| HFD | BDNF | [62,63][62][63] | |
| IL-1β | |||
| TNF-α | |||
| NF-κB | |||
| Multiple Sclerosis | Iba-1 | [64,65,][64]66,[65]67,[68,69,66][67][68][69]70[70] | |
| TNF-α | |||
| IL-17 | |||
| CCL5 | |||
| CXCR4 | |||
| Th1, Th17 | |||
| ROS | |||
| Amyotrophic Lateral Sclerosis | IL-1β, IL-12, | [35,71,[3572]][71][72] | |
| IFN-γ, IL-1α | |||
| C1q | |||
| ROS | |||
| COX-2 | |||
| iNOS | |||
| TNF-α |
References
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194.
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12.
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53.
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548.
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflammation 2019, 16, 108.
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096.
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153.
- Rao, J.S.; Kim, H.-W.; Kellom, M.; Greenstein, D.; Chen, M.; Kraft, A.D.; Harry, G.J.; Rapoport, S.I.; Basselin, M. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J. Neuroinflammation 2011, 8, 101.
- Mottahedin, A.; Ardalan, M.; Chumak, T.; Riebe, I.; Ek, J.; Mallard, C. Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front. Cell. Neurosci. 2017, 11, 190.
- Valcarcel-Ares, M.N.; Tucsek, Z.; Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Galvan, V.; Ballabh, P.; et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-Related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 290–298.
- Lauderback, C.M.; Kanski, J.; Hackett, J.M.; Maeda, N.; Kindy, M.S.; Butterfield, D.A. Apolipoprotein E modulates Alzheimer’s Abeta(1-42)-induced oxidative damage to synaptosomes in an allele-specific manner. Brain Res. 2002, 924, 90–97.
- GRAY, E.G.; WHITTAKER, V.P. The isolation of nerve endings from brain: An electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962, 96, 79–88.
- Lin, T.Y.; Yang, T.-T.; Lu, C.W.; Wang, S.-J. Inhibition of glutamate release by bupropion in rat cerebral cortex nerve terminals. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 598–606.
- Canas, P.M.; Duarte, J.M.N.; Rodrigues, R.J.; Köfalvi, A.; Cunha, R.A. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging 2009, 30, 1877–1884.
- Ahmad, F.; Jing, Y.; Lladó, A.; Liu, P. Chemical stimulation of rodent and human cortical synaptosomes: Implications in neurodegeneration. Cells 2021, 10, 1174.
- Picone, P.; Porcelli, G.; Bavisotto, C.C.; Nuzzo, D.; Galizzi, G.; Biagio, P.L.S.; Bulone, D.; Carlo, M.D.; Di Carlo, M. Synaptosomes: New vesicles for neuronal mitochondrial transplantation. J. Nanobiotechnology 2021, 19, 6.
- Wang, C.-C.; Hsieh, P.-W.; Kuo, J.-R.; Wang, S.-J. Rosmarinic Acid, a Bioactive Phenolic Compound, Inhibits Glutamate Release from Rat Cerebrocortical Synaptosomes through GABAA Receptor Activation. Biomolecules 2021, 11, 1029.
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385.
- Sheridan, S.D.; Horng, J.E.; Perlis, R.H. Patient-Derived In Vitro Models of Microglial Function and Synaptic Engulfment in Schizophrenia. Biol. Psychiatry 2022, 92, 470–479.
- Jhou, J.-F.; Tai, H.-C. The Study of Postmortem Human Synaptosomes for Understanding Alzheimer’s Disease and Other Neurological Disorders: A Review. Neurol. Ther. 2017, 6, 57–68.
- Györffy, B.A.; Tóth, V.; Török, G.; Gulyássy, P.; Kovács, R.; Vadászi, H.; Micsonai, A.; Tóth, M.E.; Sántha, M.; Homolya, L.; et al. Synaptic mitochondrial dysfunction and septin accumulation are linked to complement-mediated synapse loss in an Alzheimer’s disease animal model. Cell. Mol. Life Sci. 2020, 77, 5243–5258.
- Marcelli, S.; Ficulle, E.; Iannuzzi, F.; Kövari, E.; Nisticò, R.; Feligioni, M. Targeting SUMO-1ylation Contrasts Synaptic Dysfunction in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6609–6623.
- Gylys, K.H.; Fein, J.A.; Cole, G.M. Quantitative characterization of crude synaptosomal fraction (P-2) components by flow cytometry. J. Neurosci. Res. 2000, 61, 186–192.
- Hobson, B.D.; Sims, P.A. Critical analysis of particle detection artifacts in synaptosome flow cytometry. eNeuro 2019, 6, 27.
- Gulyássy, P.; Puska, G.; Györffy, B.A.; Todorov-Völgyi, K.; Juhász, G.; Drahos, L.; Kékesi, K.A. Proteomic comparison of different synaptosome preparation procedures. Amino Acids 2020, 52, 1529–1543.
- Plum, S.; Eggers, B.; Helling, S.; Stepath, M.; Theiss, C.; Leite, R.E.P.P.; Molina, M.; Grinberg, L.T.; Riederer, P.; Gerlach, M.; et al. Proteomic Characterization of Synaptosomes from Human Substantia Nigra Indicates Altered Mitochondrial Translation in Parkinson’s Disease. Cells 2020, 9, 2580.
- Gogoi, P.; Shiozaki, M.; Gouaux, E. Isolation, cryo-laser scanning confocal microscope imaging and cryo-FIB milling of mouse glutamatergic synaptosomes. PLoS ONE 2022, 17, e0271799.
- Massaro Tieze, S.; Chandra, S.S.; Vidyadhara, D.J. Subcellular Fractionation for the Isolation of Synaptic Components from the Murine Brain. J. Vis. Exp. 2022, 2022, 64574.
- Miyoshi, E.; Bilousova, T.; Melnik, M.; Fakhrutdinov, D.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.; Kawas, C.; Bohannan, R.; et al. Exosomal tau with seeding activity is released from Alzheimer’s disease synapses, and seeding potential is associated with amyloid beta. Lab. Investig. 2021, 101, 1605–1617.
- Sapp, E.; Seeley, C.; Iuliano, M.; Weisman, E.; Vodicka, P.; DiFiglia, M.; Kegel-Gleason, K.B. Protein changes in synaptosomes of Huntington’s disease knock-in mice are dependent on age and brain region. Neurobiol. Dis. 2020, 141, 104950.
- Fonseca-Ornelas, L.; Viennet, T.; Rovere, M.; Jiang, H.; Liu, L.; Nuber, S.; Ericsson, M.; Arthanari, H.; Selkoe, D.J. Altered conformation of α-synuclein drives dysfunction of synaptic vesicles in a synaptosomal model of Parkinson’s disease. Cell Rep. 2021, 36, 109333.
- Sánchez-Sarasúa, S.; Fernández-Pérez, I.; Espinosa-Fernández, V.; Sánchez-Pérez, A.M.; Ledesma, J.C. Can we treat neuroinflammation in alzheimer’s disease? Int. J. Mol. Sci. 2020, 21, 8751.
- Lanni, C.; Fagiani, F.; Racchi, M.; Preda, S.; Pascale, A.; Grilli, M.; Allegri, N.; Govoni, S. Beta-amyloid short- and long-term synaptic entanglement. Pharmacol. Res. 2019, 139, 243–260.
- Nguyen, T.P.N.; Kumar, M.; Fedele, E.; Bonanno, G.; Bonifacino, T. MicroRNA Alteration, Application as Biomarkers, and Therapeutic Approaches in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 4718.
- Obrador, E.; Salvador, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants 2020, 9, 901.
- Raiteri, L.; Raiteri, M. Synaptosomes Still Viable after 25 Years of Superfusion. Neurochem. Res. 2000, 25, 1265–1274.
- Pittaluga, A. Acute functional adaptations in isolated presynaptic terminals unveil synaptosomal learning and memory. Int. J. Mol. Sci. 2019, 20, 3641.
- Raiteri, M.; Sala, R.; Fassio, A.; Rossetto, O.; Bonanno, G. Entrapping of impermeant probes of different size into nonpermeabilized synaptosomes as a method to study presynaptic mechanisms. J. Neurochem. 2000, 74, 423–431.
- Grilli, M.; Summa, M.; Salamone, A.; Olivero, G.; Zappettini, S.; Prisco, S.D.; Feligioni, M.; Usai, C.; Pittaluga, A.; Marchi, M.; et al. In vitro exposure to nicotine induces endocytosis of presynaptic AMPA receptors modulating dopamine release in rat nucleus accumbens nerve terminals. Neuropharmacology 2012, 63, 916–926.
- Trebesova, H.; Olivero, G.; Marchi, M.; Grilli, M. The Anti-Aggregative Peptide KLVFF Mimics Aβ1-40 in the Modulation of Nicotinic Receptors: Implications for Peptide-Based Therapy. Biomedicines 2022, 10, 2231.
- Olivero, G.; Cisani, F.; Marimpietri, D.; Di Paolo, D.; Gagliani, M.C.; Podestà, M.; Cortese, K.; Pittaluga, A. The Depolarization-Evoked, Ca2+-Dependent Release of Exosomes From Mouse Cortical Nerve Endings: New Insights Into Synaptic Transmission. Front. Pharmacol. 2021, 12, 670158.
- Bilousova, T.; Melnik, M.; Miyoshi, E.; Gonzalez, B.L.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.M.; Kawas, C.; Hatami, A.; et al. Apolipoprotein E/Amyloid-β Complex Accumulates in Alzheimer Disease Cortical Synapses via Apolipoprotein E Receptors and Is Enhanced by APOE4. Am. J. Pathol. 2019, 189, 1621–1636.
- Wang, S.; Li, B.; Solomon, V.; Fonteh, A.; Rapoport, S.I.; Bennett, D.A.; Arvanitakis, Z.; Chui, H.C.; Sullivan, P.M.; Yassine, H.N. Calcium-dependent cytosolic phospholipase A2 activation is implicated in neuroinflammation and oxidative stress associated with ApoE4. Mol. Neurodegener. 2022, 17, 42.
- Pérez-Sisqués, L.; Sancho-Balsells, A.; Solana-Balaguer, J.; Campoy-Campos, G.; Vives-Isern, M.; Soler-Palazón, F.; Anglada-Huguet, M.; López-Toledano, M.-Á.; Mandelkow, E.-M.; Alberch, J.; et al. RTP801/REDD1 contributes to neuroinflammation severity and memory impairments in Alzheimer’s disease. Cell Death Dis. 2021, 12, 616.
- Marcatti, M.; Fracassi, A.; Montalbano, M.; Natarajan, C.; Krishnan, B.; Kayed, R.; Taglialatela, G. Aβ/tau oligomer interplay at human synapses supports shifting therapeutic targets for Alzheimer’s disease. Cell. Mol. Life Sci. 2022, 79, 222.
- Cefaliello, C.; Penna, E.; Barbato, C.; Di Ruberto, G.; Mollica, M.P.; Trinchese, G.; Cigliano, L.; Borsello, T.; Chun, J.T.; Giuditta, A.; et al. Deregulated Local Protein Synthesis in the Brain Synaptosomes of a Mouse Model for Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 1529–1541.
- Megur, A.; Baltriukien, D.; Bukelskien, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 1, 37.
- Kovács, R.A.; Vadászi, H.; Bulyáki, E.; Török, G.; Tóth, V.; Mátyás, D.; Kun, J.; Hunyadi-Gulyás, E.; Fedor, F.Z.; Csincsi, A.; et al. Identification of Neuronal Pentraxins as Synaptic Binding Partners of C1q and the Involvement of NP1 in Synaptic Pruning in Adult Mice. Front. Immunol. 2021, 11, 599771.
- Bhatti, G.K.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Lifestyle Modifications and Nutritional Interventions in Aging-Associated Cognitive Decline and Alzheimer’s Disease. Front. Aging Neurosci. 2020, 11, 369.
- Wijasa, T.S.; Sylvester, M.; Brocke-Ahmadinejad, N.; Schwartz, S.; Santarelli, F.; Gieselmann, V.; Klockgether, T.; Brosseron, F.; Heneka, M.T. Quantitative proteomics of synaptosome S-nitrosylation in Alzheimer’s disease. J. Neurochem. 2020, 152, 710–726.
- Largo-Barrientos, P.; Apóstolo, N.; Creemers, E.; Callaerts-Vegh, Z.; Swerts, J.; Davies, C.; McInnes, J.; Wierda, K.; De Strooper, B.; Spires-Jones, T.; et al. Lowering Synaptogyrin-3 expression rescues Tau-induced memory defects and synaptic loss in the presence of microglial activation. Neuron 2021, 109, 767–777.e5.
- Griffiths, J.; Grant, S.G.N.N. Synapse pathology in Alzheimer’s disease. Semin. Cell Dev. Biol. 2022, 139, 13–23.
- Sharman, M.J.; Verdile, G.; Kirubakaran, S.; Parenti, C.; Singh, A.; Watt, G.; Karl, T.; Chang, D.; Li, C.G.; Münch, G. Targeting Inflammatory Pathways in Alzheimer’s Disease: A Focus on Natural Products and Phytomedicines. CNS Drugs 2019, 33, 457–480.
- Castelletto, V.; Ryumin, P.; Cramer, R.; Hamley, I.W.; Taylor, M.; Allsop, D.; Reza, M.; Ruokolainen, J.; Arnold, T.; Hermida-Merino, D.; et al. Self-assembly and anti-amyloid cytotoxicity activity of amyloid beta peptide derivatives. Sci. Rep. 2017, 7, 43637.
- Saliba, S.W.; Bonifacino, T.; Serchov, T.; Bonanno, G.; de Oliveira, A.C.P.; Fiebich, B.L. Neuroprotective Effect of AM404 Against NMDA-Induced Hippocampal Excitotoxicity. Front. Cell. Neurosci. 2019, 13, 566.
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and inflammation—An interesting interplay in parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 8421.
- Lin, C.H.; Hsieh, C.L. Chinese Herbal Medicine for Treating Epilepsy. Front. Neurosci. 2021, 15, 682821.
- Gorski, K.; Spoljaric, A.; Nyman, T.A.; Kaila, K.; Battersby, B.J.; Lehesjoki, A.E. Quantitative Changes in the Mitochondrial Proteome of Cerebellar Synaptosomes From Preclinical Cystatin B-Deficient Mice. Front. Mol. Neurosci. 2020, 13, 570640.
- Manabe, T.; Rácz, I.; Schwartz, S.; Oberle, L.; Santarelli, F.; Emmrich, J.V.; Neher, J.J.; Heneka, M.T. Systemic inflammation induced the delayed reduction of excitatory synapses in the CA3 during ageing. J. Neurochem. 2021, 159, 525–542.
- Jing, G.; Zuo, J.; Fang, Q.; Yuan, M.; Xia, Y.; Jin, Q.; Liu, Y.; Wang, Y.; Zhang, Z.; Liu, W.; et al. Erbin protects against sepsis-associated encephalopathy by attenuating microglia pyroptosis via IRE1α/Xbp1s-Ca2+ axis. J. Neuroinflammation 2022, 19, 237.
- Düsedau, H.P.; Steffen, J.; Figueiredo, C.A.; Boehme, J.D.; Schultz, K.; Erck, C.; Korte, M.; Faber-Zuschratter, H.; Smalla, K.-H.; Dieterich, D.; et al. Influenza A Virus (H1N1) Infection Induces Microglial Activation and Temporal Dysbalance in Glutamatergic Synaptic Transmission. MBio 2021, 12, e01776-21.
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front. Cell. Neurosci. 2019, 13, 509.
- Han, T.K.; Leem, Y.H.; Kim, H.S. Treadmill exercise restores high fat diet-induced disturbance of hippocampal neurogenesis through β2-adrenergic receptor-dependent induction of thioredoxin-1 and brain-derived neurotrophic factor. Brain Res. 2019, 1707, 154–163.
- Marte, A.; Cavallero, A.; Morando, S.; Uccelli, A.; Raiteri, M.; Fedele, E. Alterations of glutamate release in the spinal cord of mice with experimental autoimmune encephalomyelitis. J. Neurochem. 2010, 115, 343–352.
- Di Prisco, S.; Merega, E.; Lanfranco, M.; Casazza, S.; Uccelli, A.; Pittaluga, A. Acute desipramine restores presynaptic cortical defects in murine experimental autoimmune encephalomyelitis by suppressing central CCL5 overproduction. Br. J. Pharmacol. 2014, 171, 2457–2467.
- Vallarino, G.; Salis, A.; Lucarini, E.; Turrini, F.; Olivero, G.; Roggeri, A.; Damonte, G.; Boggia, R.; Mannelli, L.D.C.; Ghelardini, C.; et al. Healthy Properties of a New Formulation of Pomegranate-Peel Extract in Mice Suffering from Experimental Autoimmune Encephalomyelitis. Molecules 2022, 27, 914.
- Chanaday, N.L.; Vilcaes, A.A.; de Paul, A.L.; Torres, A.I.; Degano, A.L.; Roth, G.A. Glutamate release machinery is altered in the frontal cortex of rats with experimental autoimmune encephalomyelitis. Mol. Neurobiol. 2015, 51, 1353–1367.
- Vilcaes, A.A.; Furlan, G.; Roth, G.A. Inhibition of Ca2+-dependent glutamate release from cerebral cortex synaptosomes of rats with experimental autoimmune encephalomyelitis. J. Neurochem. 2009, 108, 881–890.
- Fernández Hurst, N.; Chanaday, N.L.; Roth, G.A. GABAergic Agonists Modulate the Glutamate Release from Frontal Cortex Synaptosomes of Rats with Experimental Autoimmune Encephalomyelitis. Inflamm. Allergy Drug Targets 2015, 14, 105–110.
- Di Filippo, M.; De Iure, A.; Giampà, C.; Chiasserini, D.; Tozzi, A.; Orvietani, P.L.; Ghiglieri, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; et al. Persistent activation of microglia and NADPH drive hippocampal dysfunction in experimental multiple sclerosis. Sci. Rep. 2016, 6, 20926.
- Ravera, S.; Torazza, C.; Bonifacino, T.; Provenzano, F.; Rebosio, C.; Milanese, M.; Usai, C.; Panfoli, I.; Bonanno, G. Altered glucose catabolism in the presynaptic and perisynaptic compartments of SOD1G93A mouse spinal cord and motor cortex indicates that mitochondria are the site of bioenergetic imbalance in ALS. J. Neurochem. 2019, 151, 336–350.
- Raiteri, L.; Paolucci, E.; Prisco, S.; Raiteri, M.; Bonanno, G. Activation of a glycine transporter on spinal cord neurons causes enhanced glutamate release in a mouse model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2003, 138, 1021–1025.
