2. Biopolymer-Based Nanomaterials for Successful Wound Treatment
Organic compounds generated by living organisms are known as natural polymers or biopolymers
[24][59]. An amino acid, monosaccharide, nucleoside, or ester can be made into a peptide, a polysaccharide, a polyphenol, and a polyester by covalently bonding these units together. Based on their properties, biopolymers from plants, animals, fungi, and bacteria can be used. Due to their biocompatibility, biodegradability, decreased antigenicity, and recyclability, these polymers outperform synthetics in many applications
[25][60]. Due to their effective antibacterial, anti-inflammatory, and proliferative properties, numerous biopolymers, such as collagen, cellulose, chitosan, alginate, hyaluronan, and carrageenan, have been widely employed for wound healing. These biopolymers have been developed into a new class of wound dressings using nanotechnology-based engineering methodologies
[26][61]. Polymers derived from living organisms, such as bacteria, plants, and algae, have material properties that make it easy to mold them into hydrogels, scaffolds, and blends with other polymers to produce a skin substitute with enhanced mechanical strength, biomimetic characteristics, as well as other desirable characteristics
[27][62].
Many biopolymers, which are polymers made by live microorganisms, are utilized in the treatment of wounds. Despite this, It looks as though some polymers receive increased awareness as wound dressings than others
[28][63]. Wound dressings must be made to enhance and speed up the process of recovery. This can be done by shielding the wound from things like contamination and moisture loss, which could slow or impede healing
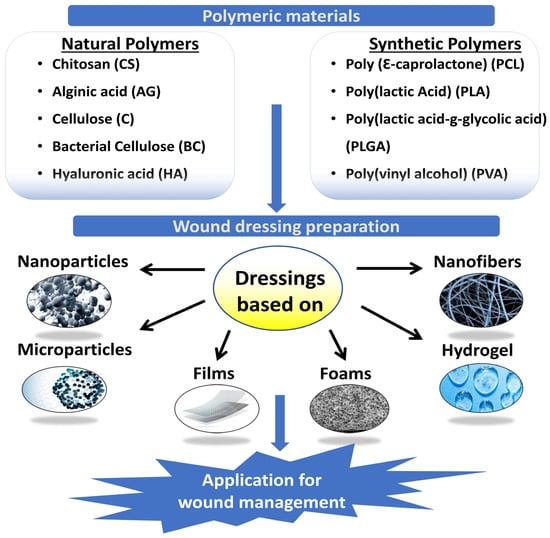
[18]. Natural and synthetic polymers and their mixtures are employed in the wound dressings′ films, and sponges. Fibers or hydrogel-based hydrogels (
Figure 1). In an ideal wound dressing, ECM of the skin would match the structure and biological features of the dressing. Due to their low cost, non-toxicity, and environmental friendliness, polymers found in nature are preferred for wound treatment over manufactured polymers
[29][64]. Polysaccharides are natural polymers commonly used as wound dressings
[30][65].
Figure 1. Polymeric materials for wound management.
The morbidities associated with peritoneal adhesions after surgery include infertility, pain, and bowel obstruction. The researchers Ito et al. (2007) developed new injectable hydrogels based on dextran (DX) using hydrazide-modified carboxymethyldextran (CMDX–ADH) and aldehyde-modified DX (DX–CHO) or carboxymethylcellulose (CMC–CHO). A CMDX–DX gelling resulted in shrinkage, whereas a CMDX–CMC gelling resulted in swelling. Treatment of mesothelial cells and macrophages with CMDX-ADH and CMC-CHO, their cytotoxicity was minimal to mild, while that of DX-CHO was extremely high. The cytotoxicity of all cross-linked gels, however, was very mild. The CMDX–CMC formula significantly reduced the formation of adhesions in the rabbit sidewall defect-bowel abrasion model, while the CMDX–DX formula significantly worsened adhesion formation. Peritoneal adhesions were effectively prevented with cross-linked hydrogels of CMDX-ADH and CMC-CHO. There are potential advantages in preventing peritoneal adhesions with dextran-based hydrogels because they degrade slowly and are relatively inexpensive
[31][66].
Chen et al. (2021) reported improved wound healing and anti-inflammation effectiveness by using GelMA/OD/Borax hydrogel as a hemostatic and anti-inflammatory hydrogel. GelMA was synthesized by modifying gelatin with methacrylic anhydride (MA) and polymerizing the polymer with UV light. Dextran′s o-hydroxyl groups are oxidized, producing aldehyde groups that bind with the -NH2 on tissue surfaces, forming the bonds that hold the tissues together. Additionally, the tri-network hydrogel showed excellent hemostatic capacity and was capable of withstanding high blood pressure of 165 mmHg, which exceeds the threshold for adults (120 mmHg). Hydrogels were effective in blocking bleeding even when used with Borax despite their mechanical properties, morphology, biocompatibility, and degradation. An innovative design modality has been used to develop a multifunctional hemostatizing hydrogel that can effectively stop bleeding and heal wounds
[32][67].
Massive bleeding poses a threat to patient safety, which makes it a challenging clinical problem. In order to promote wound healing and repair, it is imperative to develop something with hemostatic and antibacterial capabilities. Xie et al. (2022) developed a multifunctional hydrogel containing carboxymethyl chitosan (CMCS)/sodium alginate (SA)/oxidized dextran (ODE) (CSO). In order to achieve fast hemostasis, the hydrogel used Schiff base and amide reactions in order to gel rapidly, adhere effectively to the wound site, and gel quickly.
Staphylococcus aureus wound infections can be prevented with the hydrogel since CMCS and ODE possess antibacterial properties. A CSO hydrogel induced wound healing in mice by retaining water and mimicking the three-dimensional structure of the natural extracellular matrix. An in vitro whole-blood clotting assay demonstrated that red blood cells adhere well to hydrogel, resulting in good hemostasis after liver injury and tail amputation in rats. The biocompatibility of CSO hydrogel greatly improves hemostasis and wound healing
[33][68].
An in situ cross-linking method was devised by Chen et al. (2022) to prepare carboxymethyl chitosan (CMCS)/oxidized dextran (OD)/polymeric glutamine acid hydrogels that promote hemostasis and antimicrobial activity. In the COP hydrogel, γ-PGA performed the function of draining the wound surface moisture and enhancing the adhesion with the surrounding tissues. Moreover, γ-PGA and CMCS can both electrostatically adsorb negative red blood cells and concentrate blood by absorption plasma. The antibacterial properties of CMCS and OD were imparted to COP hydrogel as a result of their antibacterial properties. The inhibition zone experiment demonstrated a clear boundary around the COP hydrogel. A study conducted in vivo demonstrated that COP hydrogel inhibited bacterial growth and promoted wound healing. A diffuse hemorrhage wound was hemostasized with COP hydrogel in a rat tail model. The multifunctional COP hydrogel is expected to be used in a variety of applications in order to achieve wound hemostasis and healing
[34][69].
2.1. Collagen
Collagen is a common mammalian protein and an important part of ECM. Their triple helix structure is similar to that of collagen fibrils in terms of chemistry, with each fibril being a polymer made up of repeated amino acids linked together by peptides. The most prevalent collagen types in connective tissue are I, II, and III. In most conditions, collagen and proteolytic cleavage fragments, such as gelatin, help repair tissue due to their 29 different types
[35][70].
Components such as type I collagen are essential for tissue healing. Numerous proteolytic enzymes are produced and cleave collagen into small parts during the inflammatory phase following damage. They are chemotactic to macrophages and mitogenic to fibroblasts, which leads to the production of granulation tissue and proliferation in the cells
[36][71]. Arginylglycylaspartic acid (RGD) sequences are found in these fragments. A similar transition occurs between epithelium and mesenchyme in keratinocytes in the epithelium, facilitating tissue migration
[37][72]. The delicate balance between collagen synthesis and breakdown determines whether or not a wound will heal properly. Because of the defective MMP/tissue inhibitors of metalloproteinase (TIMP) ratio, the newly generated collagens are split into fragments in chronic wounds, prolonging the inflammatory phase
[38][73].
Increased surface modification, nanoscale reduction in collagen size, depolymerization, and labeling with anti-inflammation and anti-microbial chemicals can all increase the physiologic function of collagen
[39][74]. Electrospinning, scaffold building, and combining biopolymers with medicinal compounds can improve their physical, mechanical, and functional properties. The ECM′s native collagen has closely resembled with electrospun collagen nanofibres coated with laminin which improves keratinocyte adhesion and migration
[40][75]. Using silver oxide nanoparticles in methylcellulose hydrogels, Kim et al. (2018) developed a burn wound treatment option that enhances healing. By scavenging reactive oxygen species and promoting re-epithelialization, both quercitin and curcumin promoted diabetic wound healing in different experimental setups. In addition to sponges, hydrogels, films, membranes, powders, and freeze-dried sheets, collagen-based wound healing products are commercially available. Biopad, Helix bioactive collagen, and many other products fall into this category. It is important to choose the right product for the right wound type, as well as ensuring that the product contains the right active ingredients
[41][76].
There has been an increase in the use of tissue engineering products as an alternative treatment for chronic wounds and burns. It has some disadvantages, such as additional steps and a lack of antibacterial characteristics, which might hinder wound healing; these problems must be adequately resolved for optimal wound healing
[42][83]. Salleh et al. (2022) created a functionalized dual-layered hybrid biomatrix made of gelatin/cellulose hydrogel (outer layer) combined with graphene oxide and silver nanoparticles (GC-GO/AgNP) to fight potential post-implantation extraneous bacterial infection and collagen sponge (bottom layer) to promote cell proliferation and adhesion. GENIPIN 0.1% (
w/
v) was used to crosslink the bilayer hybrid scaffold for 6 h, followed by an advanced freeze-drying technique. Bilayer bioscaffolds were evaluated for their microstructure, biodegradability, surface wettability, antibacterial activity of nanoparticles, mechanical strength, and biocompatibility. Bilayer bioscaffolds showed good results for wound healing applications because their porosity enables them to absorb water and the biodegradation rate is slow. The biomatrix was also discovered to have hydrophobic qualities which are perfect for cell adhesion and mechanical strength. Additionally, using the disk diffusion technique, the hybrid GO-AgNP demonstrated antibacterial characteristics. Human dermal fibroblasts exhibited good cellular compatibility with the biomatrix. A potential application for the fabricated bilayer scaffold is the healing of skin wounds
[43][84].
2.2. Cellulose
Most plant cell walls are made of cellulose, a biopolymer that is plentiful.
d-glucose repeat units are bound together by 1,4-glycosidic connections in this compound. In contrast, bacterial cellulose is purer and more porous than plant cellulose. Certain bacteria from the Acetobacter, Sarcinaventriculi and Agrobacterium genera also generate it
[44][85]. For the most part, the use of cellulose in wound management owes to its moisture-retentive qualities; moist injuries heal more quickly because growth factors and other healing-promoting chemicals are better supplied to the tissues. The porous cellulose structure resembles the skin′s ECM, assisting in tissue regeneration by aiding in the absorption of exudates (such as necrotic tissue and fibrinous covering)
[45][86]. Improved functional qualities of cellulose have been achieved by bioengineering techniques such as surface immobilization with medicines or medicinal compounds, polymer blending, and electrospinning. Antimicrobials, such as those found in cellulose, can help keep the wound site free of infection
[46][87]. Using myostatin, silver nanoparticles, and cellulose that has been impregnated with chloramphenicol, antibacterial activity against E. coli and Staphylococcus aureus has been documented
[44][85]. Researchers evaluated cellulose-loaded therapeutic drugs such as vacarin, povidone iodide, and minocycline for their efficacy in drug release and wound healing qualities
[47][88]. According to Lin et al. (2013) chitosan-coated bacteria-derived cellulose membranes improved epithelialization and regeneration more quickly than commercially available Tegaderm. Burn wound neovascularization and re-epithelialization were enhanced by UV-crosslinked cellulose and acrylic acid hydrogels. As a result of its nanoscale structure, nano-fibrillar cellulose has shown promising activity. Improved control of cellular responses has been demonstrated with cellulose functionalization through methylation and oxidation. Oxidized cellulose has also been found to exhibit hemostatic properties in research. Polyuronic acid is generated as a result of cellulose oxidation, and the resulting insoluble cellulose is known as oxidized cellulose. Compared to oxidized non-regenerated cellulose (ONRC), oxidized regenerated cellulose (ORC) has structured fibrils. Collagen and ORC alone, as well as when combined, both increase in vitro fibroblast proliferation
[48][89].
In rabbits, carboxymethyl cellulose enhanced corneal epithelial repair, which was mediated by Zonula occludens (ZO)-1 expression, leading to a regeneration of the corneal epithelial barrier
[49][90]. Human epidermal keratinocytes and fibroblasts were freeze-dried with bacterial cellulose and acrylic acid hydrogels to enhance recovery in incomplete thickness burn injuries. In animals with wounds, a 3D scaffold comprised of bacterial cellulose and gelatin exhibited full epithelial regeneration
[50][91]. The healing potential of cellulose was examined at various pH levels. In a cutaneous wound model, acidic cellulose increased healing characteristics. Furthermore, polydopamine, tungsten oxide, and soy protein hydrolysate-conjugated cellulose enhanced integrin 1 expression, accelerated epithelialization, and exhibited antibacterial and anti-inflammatory activities
[51][92]. In addition to retaining moisture, some cellulose-based dressings might act as a mechanical barrier and protect the delicate granulation tissue. Cellulose-based biomaterials have tremendous potential for tissue healing applications, and recent biotechnological advances have made it possible to enhance material properties to meet consumer needs
[52][93].
2.3. Alginic Acid
The linear polymer of alginic acid is composed of repeated units of 1,4 glycosidic bonds between
d-mannuronic acid and
l-guluronic acid. Laminaria, Macrocystis, and Ascophyllum species generate alginic acid in maritime environments. By keeping the wound bed moist, absorbing excretions, reducing infection burden, removing odor, and aiding in hemostasis, alginates eliminate wound discomfort
[53][94]. Alginate-containing dressings exchange calcium ions with sodium ions in blood or wound exudate when they come into contact with the exudate. The alginate fiber swells, partly disintegrates, and forms a gel after calcium ions are sufficiently supplanted by sodium ions. It has been shown that alginates stimulate monocytes to produce IL-6 and TNF-, which are essential for healing
[54][95]. Alginate has poor cell adhesion capabilities on its own, but cell-interacting alginates can be synthesized by adding peptide sequences. A peptide sequence known as RGD is most commonly used for attachment, as it is recognized by integrin receptors. Alginates that resemble the structure of the ECM have been shown to increase cellular responses. When combined with several other polymers such as PVA and PCL, sodium alginate′s physical and mechanical properties have been demonstrated to be more stable. Alginate and PVA electrospun dressings have been shown to have superior properties to several commercial formulations
[55][96].
Aloe vera extract combined with sodium alginate and cross-linked with UV light significantly decreased UV transmittance and shielded wounds from light
[56][97]. Murakami et al. (2010) showed that a combination of chitosan, alginate, and fucoidan re-epithelialized a mitomycin-treated damaged wound
[57][98]. Numerous such experiments demonstrate alginate′s suitability for modification. There have been several studies showing that alginate dressings improved healing, including those with curcumin or silver nanoparticles, silk fibroin composites and chitosan-alginate dressings. The freeze-dried composite of cellulose, chitosan, and alginate enhanced fibroblast and endothelial cell motility, acted as an anti-seawater barrier, and boosted EGF, bFGF, and CD31 expression in wounds with a full thickness
[58][99]. Rats recovered from wounds faster when an EGF-loaded hydrogel was composed of N-carboxymethylchitosan and alginate. It was found that zinc crosslinked alginate showed enhanced epithelialization in rats compared with other divalent crosslinkers such as calcium, zinc, and copper
[59][80]. Alginate is the active ingredient in a number of wound dressings, including 3M Tegaderm, Algicell, Algisite, Calcicare, Cutimed alginate, Dermalginate, Kaltostat, and Nu-derm. All of them are unique in terms of active substance composition and extra alterations made to fulfill the needs of particular wound types
[60][100].
2.4. Hyaluronic Acid
Skin naturally contains glycosaminoglycans like hyaluronic acid. Evolution has conserved this biopolymer in mammalian cells. It consists of repeated units of N-acetyl-
d-glucosamine and d-glucuronic acid that are linked by changing 1,4 and 1,3 glycosidic bonds. Hyaluronic acid is a chemical with a high molecular weight (5106 Da)
[61][101]. Hyaluronan with a large molecular mass is anti-inflammatory, whereas fragmented hyaluronan has a pro-inflammatory effect. It is critical for wound healing because it stimulates fibroblast proliferation, ECM remodeling, and keratinocyte migration. It has been discovered that the breakdown product of hyaluronic acid has a pro-angiogenic action. Through binding to CD44 receptors on keratinocytes, it causes a cascade of events that promote differentiation
[62][102]. Exogenous hyaluronic acid has been shown to decrease scar formation and collagen synthesis after injury even though hyaluronic acid is an endogenous biopolymer. The favorable properties of hyaluronan have made it an important therapy for wound healing
[63][103]. Li et al. (2018) demonstrated that hyaluronic acid grafted with pullulan enhanced anti-enzymatic degradation. Additionally, it demonstrated faster healing due to its hemostatic activity. Furthermore, chitosan/hyaluronate hydrogels containing vancomycin microspheres increased endothelial cell proliferation at the wound site while reducing microbial burdens. When chitosan/polycaprolactone and hyaluronic acid scaffolds were electrospun with chitosan/polycaprolactone and aminoethyl methacrylate nano-hydrogels, the same effects were observed. Angiogenesis and regeneration of the dermis in diabetic ulcers were stimulated by cross-linking hyaluronan with epoxy and other layers. Applied to infected lesions, hyaluronic acid, gelatin, and chondroitin sulfate-based skin substitutes increased cell migration, reduced oxidative stress, and inhibited bacterial growth
[64][104].
A photopolymerizedglycidyl methacrylate-hyaluronic acid compound exhibited enhanced hydrogel characteristics. Hu and colleagues demonstrated that lowering TGF-1 expression created a fetal-like environment for wound healing using a three-dimensional hyaluronic acid scaffold. It was discovered that applying topical hyaluronate to gingival surgery patients speeded up the healing process and reduced pain and swelling after the procedure. In rats, a mouldable hydrogel composed of hyaluronic acid, bisphosphonate, and silver demonstrated antibacterial and self-healing properties
[65][105]. The chitosan/hyaluronate and edaravone conjugate demonstrated increased blood compatibility. Hyaluronic acid is found in commercial wound care products such as Dermaplex, Regenecare, Hyalomatrix, and Hyalofill, which provide cosmetic and protective benefits.
[56][97].
2.5. Chitosan
Chitin, common in fungus, insects, molluscs, and crustaceans, consists of 1,4-linked N-acetylglucosamine molecules. In contrast to chitin itself, chitosan is an active deacetylated derivative. It has been demonstrated that chitosan encourages hemostasis and speeds up tissue regeneration
[66][106]. Chitosan has been extensively explored as an antibacterial agent for wound infection prevention. Chitosan also promotes cell proliferation which is essential to healing, in addition to its antibacterial and antifungal properties. Polymorphonuclear leucocytes and macrophages are known to be stimulated by it for phagocytosis and the production of IL-1, TGF-, and PDGF
[67][107]. Chitosan also promotes fibroblast proliferation, and fibroblast activation is positively correlated with the level of de-acetylation
[68][108]. Hydrogels, foams, and scaffolds are made using chitosan and other polymers. When combined with other biopolymers and surface modifications, chitosan has shown promising wound healing results
[69][109].
Using chitosan nanoparticles loaded into an Ag-metalorganic framework (0.1%Ag@MOF/1.5%CSNPs) and polyvinyl alcohol/sodium alginate/chitosan (PACS) as upper and lower layers, Zhang et al. (2021) successfully prepared a bilayer composite dressing for wound healing. Bilayer dressings were evaluated for their performance. PACS had good water retention, swelling, water vapor permeability, and biocompatibility, while PACS had barely any antibacterial activity. Antibacterial activity of the upper layer (Ag@MOF/CSNPs) was excellent, but biocompatibility was poor. In addition to preventing direct contact with the skin, it can also inhibit microbial growth. Moreover, the bilayer promotes blood coagulation and cell proliferation by adhering to a large number of red blood cells and platelets. CSNPs, Ag@MOF, Ag@MOF/CSNPs, and bilayer all displayed antibacterial activity in ascending order due to their synergistic antibacterial effects. A comparison of bilayer and PACS dressings in vivo revealed that the bilayer dressing accelerated wound healing significantly, whereas the PACS dressing showed less inflammation. Ultimately, this new bilayer composite dressing accelerates wound healing
[70][110].