Over the past few decades, the field of cancer therapy has seen a significant change in the way in which formulations are designed and developed, resulting in more efficient products that allow us to ultimately achieve improved drug bioavailability, efficacy, and safety. However, although many formulations have entered the market, many others have fallen by the wayside leaving the scientific community with several lessons to learn.
- translational medicine
- drug delivery
- nanomedicine
- microspheres
- implants
- medical devices
- peptide-based therapy
- chemotherapy
- radiotherapy
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
1. Introduction
Cancer is one of the most common causes of morbidity and mortality worldwide. Of all the types of cancer, lung cancer is the most commonly diagnosed cancer, making up 11.6% of all cases, and the leading cause of cancer-related death (18.4% of all cancer deaths), closely followed by female breast cancer and colorectal cancers in terms of incidence and mortality, respectively [1].
Cancer therapy has been able to overcome different challenges over time. A lot of resources have been invested in developing new therapeutic agents and setting up secure and effective treatments [2][3][2,3]. Of the available therapeutic options for cancer treatment, the most common options used nowadays are surgery, peptide-based therapy, chemotherapy, and radiotherapy [4]. These can be used as a first-line treatment in advanced cancers or in combination as either a neoadjuvant or an adjuvant treatment. As a neoadjuvant therapy, peptides, chemotherapeutics, and radiotherapeutics are administered prior to surgery to reduce the size of the tumor. As an adjuvant therapy, the administration occurs after surgery to lower the risk of cancer recurrence.
The mechanism of action differs depending on the type of therapy. Peptides aim to interfere with biological processes to stop or slow down the growth of cancer and possess many advantages, such as high specificity, low toxicity, and good biocompatibility [5][6][5,6]. The best classical example of the application of peptides in cancer treatment is the use of gonadotropin-releasing hormone (GnRH) agonists. The prolonged activation of GnRH receptors by GnRH agonists leads to desensitization through receptor downregulation to reduce the secretion of gonadotropin hormones, which results in inhibition of the growth of certain hormone-dependent tumors [7]. The main drawbacks of peptides are the extremely low half-life and the low bioavailability once administered. Therefore, in order to maintain an effective therapeutic plasma concentration, the frequency of doses needs to be increased, which inevitably leads to poor patient compliance, potential side effects, and an increase in the cost of therapy [8][9][8,9].
Chemotherapeutics focus mainly on the interruption of cell division in cancer cells using cytotoxic agents. Depending on the type of mechanism of action and chemical structure, chemotherapeutics are classified into different groups, namely alkylating agents (e.g., carmustine and cisplatin), antimetabolites (e.g., cytarabine), antitumor antibiotics (e.g., daunorubicin and doxorubicin), topoisomerase inhibitors (e.g., irinotecan), and mitotic inhibitors (e.g., docetaxel, paclitaxel, and vincristine). The main inconvenience of this type of treatment is the nonspecific distribution and inability to exclusively target cancer cells, which account for their side effects, such as gastrointestinal tract lesions, a compromised immune system, hair loss, nausea, and cardiotoxicity. These side effects lower their therapeutic index and ultimately limit the dose that can be administered to patients, often resulting in treatment failure and cancer relapse [10].
Finally, there are two common procedures regarding radiotherapy. The first is external beam radiotherapy, in which damage to cancer cells is induced by the application of ionizing radiation using an external device. The second is brachytherapy, in which a radioactive source is inserted into the body to deliver the dose to a small, well-defined anatomy [11]. Similar to chemotherapy, the lack of tumor selectivity is generally associated with short-term toxicity and long-term consequences that are very similar to chemotherapy'’s side effects. For instance, acute toxicity, such as mucositis, generally heals within weeks to months while late-onset effects such as fibrosis are generally considered to be irreversible and progress over time [12].
2. 1990s: The First Steps
During this period, two different formulation strategies aimed at increasing the therapeutic index of cancer therapies received market approval. On the one hand, extended-release formulations were designed to accurately control release rates over prolonged periods of time, achieving optimal drug concentration–time profiles and, therefore, a lower frequency of administration and less undesirable effects caused by the fluctuation in drug levels in the blood [13][14][13,14]. During this decade, various biomolecules were successfully encapsulated within subcutaneous or intramuscular long-acting formulations such as microparticles or implants (Table 1).
Table 1.
|
Type of Therapy |
Formulation Type |
Mechanism of Action |
Drug Substance |
Trade Name |
Administration Route |
Dosing Frequency |
Indications |
First Approval |
Current Status |
|
Peptide-based therapy |
Microspheres |
Inhibition of gonadotropin secretion |
Triptorelin acetate |
Decapeptyl |
IM |
1–6 months |
Prostate cancer |
1986 |
Active |
|
Microspheres |
Inhibition of gonadotropin secretion |
Leuprolide acetate |
Lupron Depot |
,23]. The size of colloid carriers dictates their in vivo fate following intravascular administration. In this respect, while colloids above 5 μm in size can cause the occlusion of blood vessels, the size of carriers in the nanometer range determines their plasma circulation time, since particle size positively correlates with the extent of recognition by the reticuloendothelial system (RES). As a result, research efforts started to focus on accurately controlling the size of colloid carriers depending on the therapeutic aim. Microspheres pursuing embolization (the action of deliberately obstructing the blood flow in a particular vessel) have been approved for the treatment of hepatocellular carcinoma. Nanomedicines that take advantage of the EPR effect have been approved for other types of cancer,
During this decade, 55% of all formulations that received authorization were peptides, which were formulated mainly within microspheres and implants. Thirty-six percent (36%) were chemotherapeutic agents formulated mainly in liposomes (Figure 1).
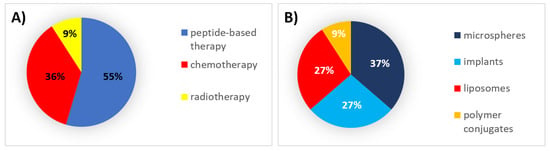
Figure 1. (A) Analysis of drug products and medical devices approved in the 1990s classified by the type of therapy. (B) Analysis of drug products and medical devices approved in the 1990s classified by the type of formulation.
3. 2000s: The Field Matures
From the beginning of the 21st century, the field matured through the diversification of new technologies in the direction of the strategies described in the previous section. Concerning the development of extended-release platforms, two distinct nonbiodegradable solid implants able to prolong peptide release over a year (including the first osmotic-driven technology) were marketed during this decade (Table 2). Moreover, the Atrigel® technology entered the arena as an alternative to solid implants, which forms implants in situ upon phase separation by solvent exchange [24][51]. With regard to delivery systems for the modification of the biodistribution of cancer therapeutics, new intravascular carrier-based technologies, such as nanoparticle albumin-bound (nab) technology, were introduced to improve the efficiency of nanoparticles and further reduce the side effects of chemotherapy (Table 2) [25][26][57,58]. Furthermore, the first microspheres for chemoembolization were CE-marked during this decade. Chemoembolization refers to the technique of injecting chemotherapeutic agents into the feeding arteries of a tumor along with particles designed to cause embolization. Transarterial chemoembolization (TACE) is a first-line treatment for the intermediate stage of HCC [27][59]. However, the possibility of the diffusion of and systemic toxicity due to the chemotherapeutics used in solution in conventional TACE [28][29][60,61] required the development of drug-eluting microspheres (DEM) as better embolization agents in TACE (DEM-TACE), as they allow for chemotherapeutics to be retained selectively in HCC.
Table 2. Formulations for cancer therapy authorized between 2000 and 2009 grouped by the type of therapy. SC: subcutaneous; IV: intravenous; IA: intra-arterial. * Conformité Européenne (CE)-marked for loading with doxorubicin or irinotecan.
|
Type of Therapy |
Formulation Type |
Mechanism of Action |
Drug Substance |
Trade Name |
Administration Route |
Dosing Frequency |
Indications |
First Approval |
Current Status |
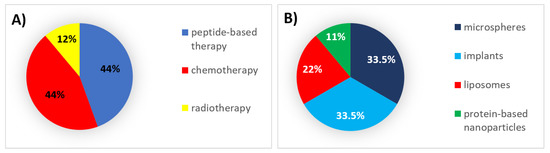
Figure 2. (A) Analysis of drug products and medical devices approved during the 2000s classified by the type of therapy. (B) Analysis of drug products and medical devices approved during the 2000s classified by the type of formulation.
4. 2010s: The End of the Beginning
During this decade, the application of previously authorized technologies was consolidated into distinct cancer therapeutics (Table 3). Concerning the development of extended-release platforms during this decade, new approvals were limited to a single solid implant and to a polymer conjugate.
Table 3. Formulations for cancer therapy authorized between 2010 and 2019 grouped by the type of therapy. SC: subcutaneous; IV: intravenous; IA: intra-arterial. IT: intratumoral; * CE-marked for loading of doxorubicin or irinotecan, ** CE-marked for loading with doxorubicin, irinotecan, idarubicin, or epirubicin.
|
Type of Therapy |
Formulation Type |
Mechanism of Action |
Drug Substance |
Trade Name |
Administration Route |
Dosing Frequency |
Indications |
First Approval |
Current Status |
||||||||||
|
Peptide-based therapy |
|||||||||||||||||||
|
Peptide-based therapy |
Solid implant |
Solid implant Inhibition of gonadotropin secretion |
Leuprolide acetate |
Viadur |
SC |
12 months |
Inhibition of gonadotropin secretion Prostate cancer |
Leuprolide acetate 2000 |
Discontinued |
||||||||||
Leptoprol | SC |
3 months |
Prostate cancer |
2015 |
Active |
IM |
1–6 months |
Prostate cancer |
In-situ-forming implants |
||||||||||
|
Polymer conjugate | Inhibition of gonadotropin secretion |
l-asparaginase depletion Leuprolide acetate |
Eligard |
SC | 1989 |
Calaspargase pegol 1–6 months |
Asparlas Prostate cancer |
2002 Active |
|||||||||||
Active | |||||||||||||||||||
IV, IM | 3 weeks |
Acute lymphoblastic leukemia |
2018 |
Active |
Solid implant |
Inhibition of gonadotropin secretion |
Goserelin acetate |
Zoladex |
SC |
Solid implant |
|||||||||
|
Chemotherapy |
Inhibition of gonadotropin secretion |
Liposomes Histrelin acetate |
Microtubule inhibition Vantas |
SC |
Marqibo 1–3 months |
12 months Prostate and breast cancer |
1989 |
Vincristine |
Prostate cancer Active |
||||||||||
IV | 1 week | 2004 |
Acute lymphoblastic leukemia Active |
2012 |
Active |
Polymer conjugate |
l-asparagine depletion |
Pegaspargase |
Oncaspar |
IV, IM |
|||||||||
2 weeks | |||||||||||||||||||
|
Liposomes |
Microspheres Immunomodulation |
- Mifamurtide |
Various* Mepact | Lymphoblastic leukemia |
Embozene TANDEM IV |
IA 1994 |
1/2–1 week Active |
||||||||||||
- | Osteosarcoma | 2009 |
Active |
Hepatocellular carcinoma |
2012 |
Active |
Solid implant |
Inhibition of gonadotropin secretion |
Buserelin acetate |
Suprefact Depot |
SC |
2–3 months |
Prostate cancer |
||||||
|
Chemotherapy |
Liposomes |
Topoisomerase-II inhibition, DNA intercalation |
Doxorubicin |
Myocet | 1996 |
IV |
|||||||||||||
|
Liposomes | 3 weeks | Metastatic breast cancer | Active |
||||||||||||||||
Topoisomerase I inhibition | 2000 |
Irinotecan |
Onivyde | Active |
IV |
2 weeks |
Metastatic adenocarcinoma of the pancreas |
2015 |
Active |
Microspheres |
Inhibition of secretion of peptides from the endocrine gastrointestinal system |
||||||||
|
Microspheres |
- |
Octreotide acetate |
Various* Sandostatin LAR |
IM |
1 month |
Neuroendocrine tumors |
1998 |
||||||||||||
|
Microspheres |
- |
Various** DC Bead |
Life Pearl IA |
- |
IA Hepatocellular carcinoma |
2003 |
- |
Hepatocellular carcinoma Active |
|||||||||||
2015 | Active | Active |
Chemotherapy |
Liposomes |
Topoisomerase-II inhibition, DNA intercalation |
||||||||||||||
|
Liposomes |
DNA polymerase inhibition + topoisomerase-II inhibition, DNA intercalation | Doxorubicin |
Doxil |
IV |
3–4 weeks |
AIDS-related Kaposi | |||||||||||||
|
Protein-based nanoparticles |
Microtubule inhibition |
Paclitaxel |
Cytarabine + daunorubicin Abraxane |
IV |
1–3 weeks | ' | ’s sarcoma, ovarian, and breast neoplasms, multiple myeloma |
1995 |
Active |
||||||||||
Breast neoplasms, pancreatic neoplasms, non-small-cell lung cancer |
Vyxeos | 2005 |
IV |
2 days |
Acute myeloid leukemia |
2017 Active |
Active |
Liposomes |
Topoisomerase-II inhibition, DNA intercalation |
||||||||||
|
Microspheres | Daunorubicin |
Daunoxome |
IV |
2 weeks |
|||||||||||||||
|
Microspheres | - |
- Various* |
Various* Hepasphere |
DC Bead LUMI IA |
IA - |
- AIDS-related Kaposi'’s sarcoma |
Hepatocellular carcinoma 1996 |
Discontinued |
|||||||||||
Hepatocellular carcinoma | 2005 | 2017 |
Active |
Solid implant |
DNA alkylation |
Carmustine |
Gliadel |
IC |
3 weeks* |
Malignant glioma |
|||||||||
|
Radiotherapy |
Microspheres |
Beta particle emission | 1996 |
Yttrium-90 |
SIR-Spheres |
IA Active |
|||||||||||||
Active | |||||||||||||||||||
|
Micelles |
Microtubule inhibition |
Paclitaxel | - | Metastatic liver tumors |
2002 |
Apealea |
IV |
3 weeks Active |
Ovarian cancer, primary peritoneal cancer, fallopian tube cancer |
2018 |
Active |
Liposomes |
|||||||
|
Radiotherapy |
DNA polymerase inhibition |
Microspheres Cytarabine |
DepoCyt |
Beta particle emission |
Holmium-166 ITh |
4 weeks |
Lymphomatous meningitis |
1999 |
QuiremSpheres |
IA |
- Discontinued |
||||||||
|
Radiotherapy |
Microspheres |
Beta particle emission |
Yttrium-90 |
Theraspheres |
IA |
- |
Hepatocellular carcinoma |
1999 |
Active |
On the other hand, researchers started to explore the potential of modifying the biodistribution of cancer therapeutics in order to increase the degree of accumulation at the target site and reduce adverse effects in patients [15][16][17][15,16,17]. This objective can be achieved by administering drugs directly to the tumor site using biodegradable polymer wafers, particularly when access to the target site is hindered when administered systemically, as in the case of brain tumors due to the blood–brain barrier [18][19][18,19]. Another method for controlling the biodistribution of anticancer agents is the intravascular administration of carriers that exploit the inherent characteristics of the tumor environment to increase the degree of accumulation of drugs within the target site, which is known as passive targeting. As a result of the enhanced permeability and retention (EPR) effect, therapeutic agents accumulate preferentially in neoplastic tissues when their circulation time in the bloodstream is prolonged [20]. The EPR effect is a phenomenon that describes the extravasation and accumulation of macromolecules or nanoparticles in neoplastic tissues [21]. This effect stems from both the peculiarities of the tumor'’s neovasculature, which is composed of poorly aligned and defective endothelial cells with wide fenestrations that become leaky and permeable enough to allow therapeutics to reach the tumor'’s surroundings, and the aberrant lymphatic architecture, wherein the high tissue pressure causes the drainage to be impaired, which ultimately helps to retain permeated nanoparticles and macromolecules [22][23][22
During this decade, 44% of all formulations that received authorization were peptides and chemotherapeutic agents, which were mainly encapsulated in implants and microspheres, respectively (Figure 2).
Hepatocellular carcinoma | |||||||||
2015 | |||||||||
Active | |||||||||
|
Inorganic nanoparticles |
Radio enhancer |
Hafnium oxide |
Hensify |
IT |
- |
Soft-tissue sarcoma |
2019 |
Active |
|
|
Other |
Inorganic nanoparticles |
Hyperthermia |
Iron oxide |
NanoTherm |
IT |
- |
Glioblastoma |
2010 |
Active |
With regard to delivery systems for the modification of the biodistribution of cancer therapeutics, three additional liposomal formulations received marketing authorization, including the second stealth liposome (after Doxil®'’s approval in the 1990s) and the very first liposome coencapsulating two distinct chemotherapy agents, which enables the simultaneous delivery of both drugs [30][50]. Moreover, in 2018, another milestone was achieved with the approval of the first micellar formulation for cancer therapy. Additional breakthroughs stem from the pioneering approval of inorganic-based nanoparticles. In fact, during this decade, two different products using inorganic nanoparticles were authorized as medical devices for cancer therapy: Hensify® as a radio enhancer in radiotherapy and NanoTherm® for magnetic hyperthermia [31][85].
During this decade, 58% of all formulations that received authorization were chemotherapeutic agents, for which microspheres and liposomes were the mainly used types of formulations (Figure 3).
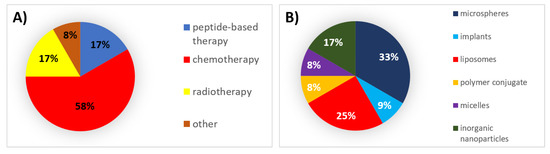
Figure 3. (A) Analysis of drug products and medical devices approved during the 2010s classified by the type of therapy. (B) Analysis of drug products and medical devices approved during the 2010s classified by the type of formulation.
5. Successes, Failures, and Lessons Learned
Over the past few decades, a wide range of innovative formulation strategies and pioneering technologies have reached the market (Figure 4) and entered into clinical trials as a result of the extensive efforts made in preclinical and clinical stages.
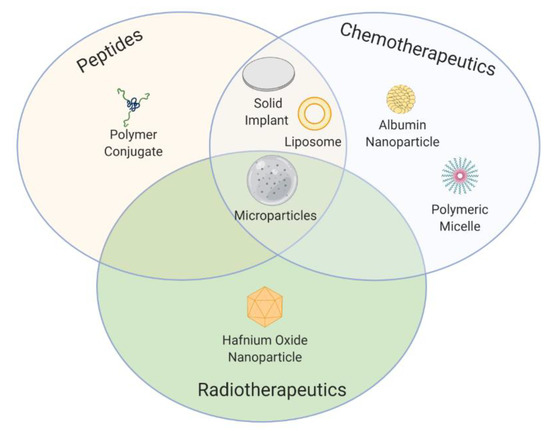
Figure 4.
Concerning peptide-based therapy, the main aim pursued with novel formulation strategies has been to overcome the short elimination half-life of peptides. In this regard, the introduction into the market of the first microspheres and implants using biodegradable polymers in the 1990s for prolonged peptide release has enabled the administration of some peptides for cancer treatment that were not clinically available (namely, goserelin) and a reduction in the frequency of administrations, improving thereby patient compliance, of some other peptides that were previously available in daily injectable formulations (such as buserelin, triptorelin, leuprolide, and octreotide). The subsequent authorization of Vantas® and Viadur® in the 2000s, which extended peptide release over a year, further reduced the frequency of administration in comparison with the previous 3–4-months'’ implants [32][168]. Unfortunately, Viadur® was discontinued due to diminished market demand and growing manufacturing costs. However, during the 2010s the innovation in microspheres and implant-based formulation technologies for peptide delivery has been abruptly reduced. It could be due to a change in tendency as 75% of all nonmarketed formulations that have entered phase III clinical trials for peptide-based therapy are polymer conjugates. However, these data should be handled cautiously, given that these phase III trials had a negative outcome, or their results are still pending.
Hormone-dependent cancers have been favored to the greatest extent by prolonged-release systems of peptide-based therapies because these cancers require continuous administration over long periods. In fact, 75% of all formulations authorized for peptide-based therapy are designed to treat hormone-dependent cancers, of which prostate cancer is the most treated [33][169]. This can be seen as a major limitation of peptide-based therapy, as these formulations are restricted to hormone-dependent cancers. This trend, however, is reversed in phase III clinical trials, as 50% of the nonmarketed formulations for peptide-based therapy are being tested for the treatment of pancreatic cancer, suggesting that the interest in using peptide-based formulations to treat other cancers is increasing. Nevertheless, the translational potential of peptide-based therapy for other types of cancers remains to be fully demonstrated, as phase III trials with peptide-based therapy for pancreatic cancer failed to meet their primary outcomes.
Concerning chemotherapeutics, the main aim pursued with novel formulation strategies has been to improve their nonspecific distribution. In this regard, various carrier-based formulations incorporating chemotherapeutics have received marketing authorization in order to reduce their toxicity and improve their therapeutic index. Ever since Doxil® was authorized in the 1990s as the first liposome formulation entrapping doxorubicin, liposomes have been trending upward in the market [34][170]. Many of them have introduced innovations such as Vyxeos® with the incorporation of two chemotherapeutics within the same formulation. In this regard, Vyxeos® may well set the foundation for future approvals exploring alternative drug combinations. In addition to their high biocompatibility, one reason why liposomes have been in the limelight over the past three decades may be the good results obtained by reducing the side effects of chemotherapy in the clinic. The reduction in the neurotoxicity using vincristine and the reduction in the cardiotoxicity of doxorubicin after encapsulation within liposomes are two representative examples [35][36][37][46,89,171] Other nanomedicines apart from liposomes have received marketing authorization for chemotherapy. This is the case of Abraxane® in the 2000s, the first nanoparticle-based formulation using albumin as a carrier and, more recently, the first micelle-based formulation entrapping paclitaxel in 2018 under the tradename Apealea®. Alternatively, during the 2000s and 2010s, microspheres containing chemotherapeutics were authorized for chemoembolization following local administration. Further improvements in these microspheres resulted in the first commercially available microsphere that combines chemotherapy with imaging function (DC Bead LUMI). Despite current research efforts, the combination of imaging and therapeutic functions remains to be achieved with nanomedicines at the clinical level.
However, the improvement of the therapeutic index of chemotherapy agents with these formulations is more related to an increase in the safety profile of the formulation than to an increase in therapeutic efficacy, which remains a challenge for the decades to come. Although the use of nanocarriers exploiting passive targeting through the EPR effect was thought to substantially improve the nonspecific distribution of chemotherapeutics, tumor accumulation remains a major hurdle as the extent of the EPR effect varies widely among patients and tumor type [34][38][170,172]. In fact, despite the wide range of preclinical studies on the pegylation strategy, only a few authorized formulations are actually stealth liposomes. Hence, it is worth noting that there currently seems to be a dilemma about whether or not the use of PEG is worthwhile [39][173]. The polymer chains that prevent the recognition of opsonins and subsequent phagocytosis by the RES may also prevent liposomes from being internalized by target cells and the PEG layer may even contribute to developing palmar–plantar erythrodysesthesia [40][174]. Later, researchers started to focus on the functionalization of the carrier with targeting moieties to exploit active targeting as an alternative to passive targeting. Nevertheless, the reality is that none of the formulations authorized during the past three decades are based on this strategy. Despite the wide range of preclinical studies on active targeting during the 2010s, its immediate clinical translation remains uncertain, as the phase III trial with the first antibody-targeted liposomal formulation (MM-302) was prematurely terminated. It seems that the high variability in receptor expression between tumors is directly related to the failure of active targeting [41][42][98,175]. Alternatively, microspheres for chemoembolization increase the selectivity of the chemotherapeutics following intra-arterial administration, but at the cost of causing discomfort to the patient, as this type of administration is a complex local invasive procedure that requires trained professionals to perform it.
Moreover, some other difficulties encountered with clinical translation are related to manufacturing issues. Platforms that require complex and/or laborious synthesis procedures generally have a limited potential for clinical translation, as they can be difficult to scale up. For example, of the 16 technologically modified formulations entrapping chemotherapeutics that have reached the market, DaunoXome® and DepoCyt® have been discontinued due to manufacturing issues.
Of all the cancers treated with chemotherapeutic agents, the most common indication is hepatocellular carcinoma (37.5%), which accounts for >80% of primary liver cancers worldwide [43][176], followed by breast cancer (19%), mostly as second-line and hematological malignancies (19%). Of the nonmarketed formulations for the delivery of chemotherapeutics that are currently being evaluated in clinical trials, breast cancer represents the most frequent indication (50%), followed by adenocarcinoma of the pancreas (37.5%). However, as it also occurred with peptide-based therapy, future trends should be analyzed with caution, given that six of these phase III trials with chemotherapeutic formulations had a negative outcome
Analogously to chemotherapeutics, concerning radiotherapeutics, the main aim pursued with novel formulation strategies has been to improve their nonspecific distribution in order to deliver the maximum amount of radiation to the tumor with minor radiation-induced damage. Microspheres containing radiotherapeutics have been authorized for radioembolization. Based on the encouraging results obtained with selective local procedures such as TACE and the lack of efficient targeting strategies observed following systemic administration, all microspheres for radiotherapy approved in the past three decades are given following intra-arterial administration. Further improvements in these microspheres resulted in the first commercially available microsphere that combines radiotherapy with imaging function (QuiremSpheres®). Alternatively, in 2019 a new class of radiation-enhancing nanoparticles formulated in crystalline HfO2 were authorized in Europe, being the first generation of radio enhancers intratumorally administered available for the treatment of solid tumors. The most common indication for formulations encapsulating radiotherapeutics is hepatocellular carcinoma (75%) followed by sarcoma (25%). As long as targeting efficiency following systemic administration is not enhanced, this type of therapy will only be suitable for accessible cancer types in which local administration is enabled. No phase III clinical trial with nonmarketed formulations of radiotherapeutics has been active in the last five years.
Figure 1, Figure 2 and Figure 3 reflect how the formulations for cancer treatment have evolved depending on the type of therapy and the type of technology used. According to Figure 1a, Figure 2a and Figure 3a, the formulations for peptide-based therapy have gone from representing 55% of the total number of new formulations approved during the 1990s to account for only 17% of the formulations approved in the 2010s. A clear parallel can be drawn between this decrease and the increase in the number of novel formulations for the delivery of chemotherapeutics, which has risen from 36% of the formulations approved during the 1990s to 58% of all formulations approved during the 2010s. The percentage of radiotherapeutics has slowly increased as well. Figure 5a shows the evolution of the approved formulations for the three types of cancer therapy to be compared. Between 2010 and 2015 the number of approved formulations for chemotherapy surpassed the number of peptide-based formulations that received authorization. Apart from the types of therapy, it is important to mention how the types of authorized formulations have evolved over time. As shown in Figure 5b, concerning the type of formulation used, both microcarriers and nanocarriers lead the marketed formulations (36.5%), whereas solid implants and polymer conjugates are lagging behind (21% and 6%, respectively). Figure 1b, Figure 2b and Figure 3b illustrate how liposomes and microspheres have been in the limelight over the past three decades while implants have been trending downwards since the 2000s.
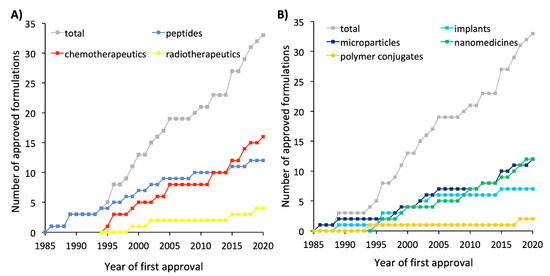
Figure 5.
A
B
Regarding the new formulation technologies that are expected to be approved in the future, active targeting, stimuli-responsive systems, and multifunctional systems are setting trends at the preclinical level. However, only MM-302, an actively targeted liposomal formulation, and ThermoDox®, a thermosensitive liposomal formulation, have reached phase III clinical trials. Hence, clinical translation of these sophisticated systems is not expected to occur in the short term. Most of the formulations that have reached phase III clinical trials are liposomes for chemotherapeutics and polymer conjugates for peptides. As a novelty, Stimuvax®, a vaccine for non-small-cell lung cancer, has completed a phase III clinical trial.
Regulatory agencies play an important role in the approval of novel formulations. Even though some of the products reviewed herein are not approved either in Europe or in the United States, 42.4% of all formulations are approved by both regulatory agencies (although approval does not necessarily occur concomitantly; e.g., Oncaspar® was approved by the EMA 22 years after being approved by the FDA). Some formulations, including Suprefact®, Mepact®, Myocet®, DC Bead®, HepaSphere®, Leptoprol®, Apealea®, LifePearl®, Embozene Tandem®, DC Bead LUMI®, QuiremSpheres®, Hensify®, NanoTherm®, and BioPearl®, are authorized for use only in Europe, and Viadur®, Vantas®, Asparlas®, and Marqibo® only in the United States. Much of this percentage mismatch is attributable to the DEM-TACE microspheres, which have been approved as medical devices in Europe and the United States, but the drug-loading feature has yet to be approved by the FDA [44][177]. Overall, drug products represent 66.7% of the total number of approved formulations for cancer therapy, whereas medical devices represent the remaining 33.3%. However, these data are not uniform among the distinct types of cancer therapy; whereas 100% of the products approved for peptide-based therapy are drug products, the trend is reversed in the case of radiotherapy, where medical devices account for 100% of the approved formulations.
In conclusion, despite the lessons learned during these three decades that have allowed us to acquire a profound technological and pharmacological understanding of the development of novel formulations that have led to important advances in the treatment of cancer, there is still a long way to go to continue to improve the field of cancer therapy.
