Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Maria Valentina Dinu.
The introduction of selective recognition sites toward certain heavy metal ions (HMIs) is a great challenge, which has a major role when the separation of species with similar physicochemical features is considered. In this context, ion-imprinted polymers (IIPs) developed based on the principle of molecular imprinting methodology, have emerged as an innovative solution. Advances in IIPs have shown that they exhibit higher selectivity coefficients than non-imprinted ones, which could support a large range of environmental applications starting from extraction and monitoring of HMIs to their detection and quantification.
- adsorption
- ion-imprinted polymers
- heavy metal ions
- selectivity
1. Introduction
The removal and monitoring of heavy metal ions (HMIs) from/in aqueous environments are continuously in demand because of their high persistence in ecosystems, ability to accumulate in living organisms, and high toxicity [1,2][1][2]. In the past few years, numerous attempts have been reported to minimize the harmful impact of HMIs [3,4,5,6,7,8][3][4][5][6][7][8]. In this regard, various water treatment technologies, including liquid–liquid extraction, solid–liquid extraction (or solid-phase extraction, SPE) [3[3][4][5][6][8],4,5,6,8], coagulation/flocculation [3[3][4][5][9],4,5,9], chemical precipitation [3,4[3][4][5],5], oxidation [3[3][4][5],4,5], bioremediation [3[3][4][5][10],4,5,10], electrochemical treatment [3,4[3][4][5],5], and membrane filtration technologies [3,4,5,11][3][4][5][11] have received increasing attention.
Sorbents bearing various chelating ligands were prepared by two main strategies: (i) post-functionalization of commercially available [12,13,14,15,16][12][13][14][15][16] or homemade [12,17,18,19,20][12][17][18][19][20] cross-linked copolymers; (ii) “bottom-up” design of specialized materials by the free radical polymerization of monomers endowed with adequate pre-existing heteroatomic moieties, such as –COOH, –OH, –SH, –NH2, =NH, –N=, –SO3H, and –PO3H, in the presence of a cross-linker [18,20[18][20][21],21], or by the condensation of monomers containing a high density of reactive functional groups [12,22][12][22]. Even so, all the sorbents described above have weak selectivity.
Although selective recognition is a challenging issue for both wastewater treatment and HMIs monitoring, it is laborious to accomplish with the above-mentioned sorbents. The development of highly selective sorbents for the purification of wastewater and recovery of HMIs requires a multitude of recognition sites and tailored morphologies [23]. To achieve these goals, molecular imprinting was developed as an emerging technology to create selective recognition sites in a polymeric matrix [24,25,26,27][24][25][26][27]. Molecularly imprinted polymers (MIPs) are well-known as the synthetic mimics of biological recognition systems, such as antibody–antigen, enzyme–inhibitor, and receptor–effector [26,27][26][27]. The operation principle of MIPs is based on a “lock and key” mechanism similar to that of an antibody that is capable of recognizing a specific antigen in the biological system. Compared with natural receptors, MIPs have the advantages of long-term stability, low cost (cheap starting materials), and better control of the response (stimuli-responsive MIPs) [6,28][6][28]. Consequently, MIPs have found a fast-growing trend in analytical chemistry (chromatography, SPE, and chiral separations) as well as in medicine, sensing, and catalysis [24,25,26,27,28][24][25][26][27][28].
The concept of MIPs has also been considered to design ion-imprinted polymers (IIPs). The first study on IIPs was published by Nishide et al., in 1976 [29], who synthesized IIPs by cross-linking poly(4-vinylpiridine) [P(4-VP)] with 1,4-dibromobutane in the presence of a metal ion as a template (such as Co(II), Cu(II), Fe(III), Hg(II), Ni(II), or Zn(II)). Nevertheless, a considerable interest in the development of this field is more recently visible as the number of publications has steadily increased year-by-year (Figure 1). In addition, since 2013, the preparation of composite IIPs for the selective separation of HMIs has also received growing attention from the scientific community (Figure 1).
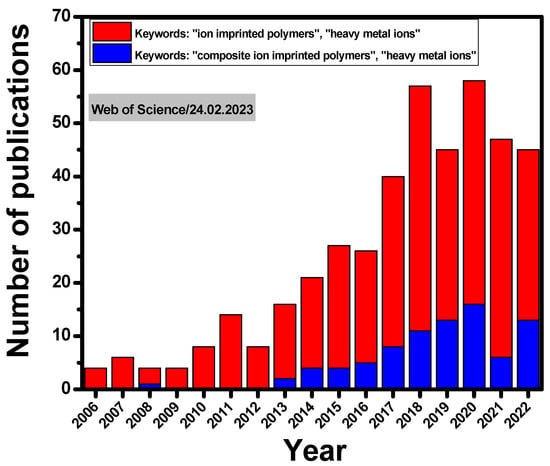
Figure 1. A timeline showing the number of publications on IIPs and composite IIPs in the last 16 years indexed by the Web of Science™ database.
IIPs exhibit similar features as MIPs, the main difference being related to the specific recognition sites that are inorganic ions after the imprinting process in the case of former ones [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38]. The strategies applied to prepare MIPs have been also adapted to the synthesis of IIPs and they will be briefly described in the next section. However, all of the approaches follow a similar outline, as can be seen in Figure 2, where the imprinting process using functional monomers as a ligand is depicted.
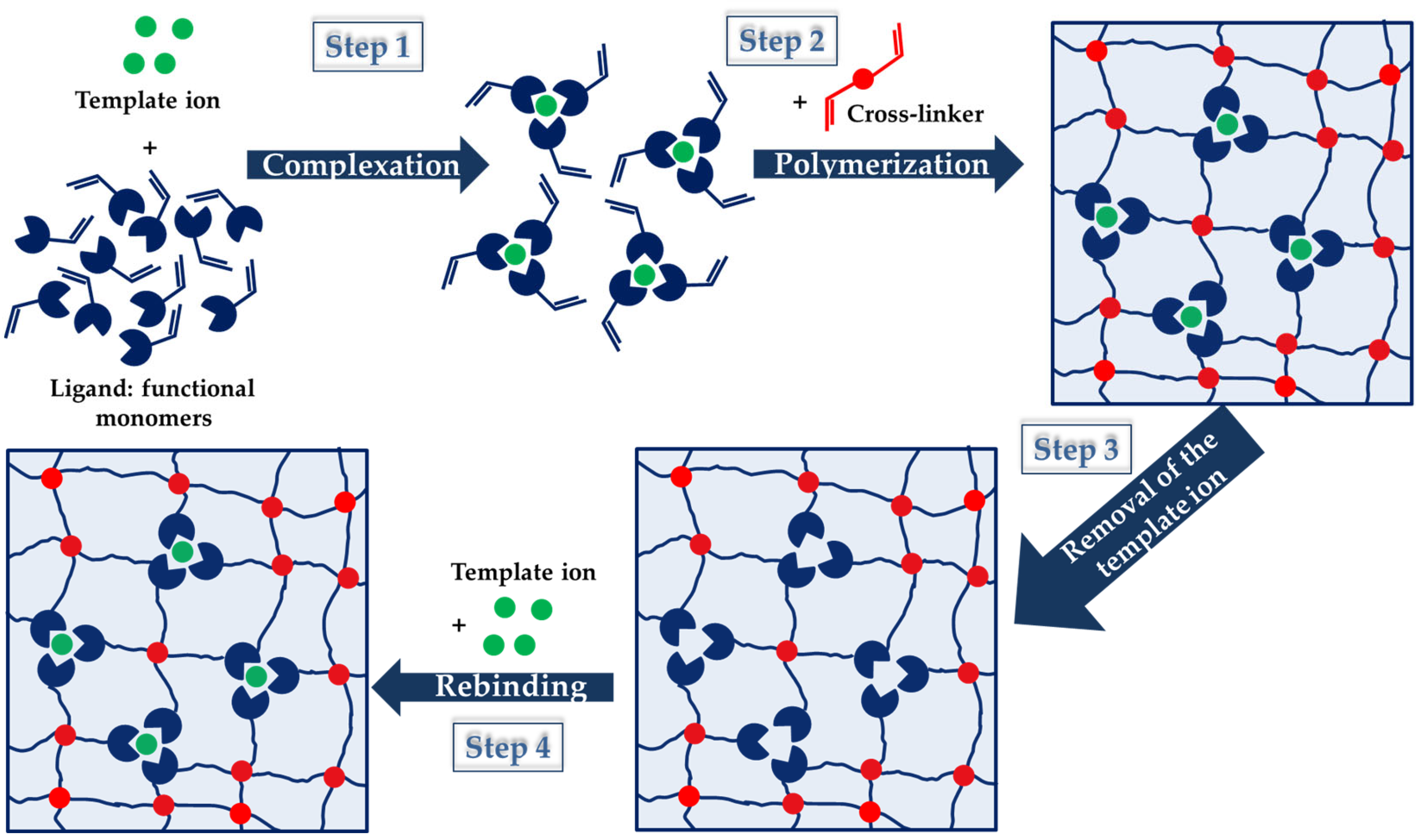
Figure 2. A schematic representation of the steps employed within the IIPs synthesis. The imprinting process is presented using functional monomers as ligand.
As Figure 2 shows, the IIP synthesis is based on three steps: (i) in the first step, a complex between metal ion (template) and ligand functional groups of the host (monomers) is generated by non-covalent interactions (chelation, electrostatic interactions, and hydrophobic interactions); (ii) in the second step, the polymerization of this complex and the stabilization of the binding cavities are achieved using a bi-functional monomer (cross-linker); (iii) in the third step, the template ion is leached from the copolymer host using adequate compounds, and thus specific cavities available for selective rebinding are created [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38]. To prove the existence of pre-organized recognition sites, in the fourth step (Figure 2), the IIPs are exposed to the template ion, and the imprinted cavities are thus selectively filled by the target metal ion [38]. Lately, driven by the intrinsic features of polysaccharides, the cross-linking of natural polymers, including chitosan (CS), cellulose (CEL), and alginate (ALG), carrying metal-binding groups has been the main approach used to engineer IIPs [33,34,37,38][33][34][37][38].
2. Important Features on IIPs’ Synthesis and Evaluation of Selective Binding Properties
2.1. Main Components of IIPs
The main components of IIPs, besides the target ion that is the template, are generally the functional monomers, cross-linkers, solvents (porogens), and initiators. The selection of these compounds is crucial for the preparation of IIPs with high selectivity and specificity [38].
2.1.1. Templates
As presented above for the preparation of IIPs, metal ions are usually the templates. The selectivity of IIPs is diminished when a target metal ion exhibits similar physicochemical characteristics to the competing ones. Thus, during the preparation process, the template ion and its ligand are adequately chosen to improve IIP selectivity [38].
2.1.2. Functional Monomers and Polymers
In the selection of functional monomers, the following aspects should be considered: (i) the monomer should contain functional moieties that can bind the template ion; (ii) the monomer should be stable during the polymerization process; and (iii) the monomer should not contain ligand groups that inhibit polymerization. Commercial monomers that contain a vinyl group such as methacrylic acid (MAA), 4-vinylpyridine (4-VP), 1-vinylimidazole (1-VI), acrylamide (AAm), and acrylic acid (AA) were mainly employed as ligands to obtain IIPs [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38]. However, these monomers have low efficiency in terms of selectivity. When the ligands did not contain vinyl groups, they were modified to include polymerizable moieties, as in the case of N-methacryloyl-L-histidine (MAH) and crown ethers [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38]. Furthermore, the introduction of the trapping approach by Rao et al. [30] has allowed the physical entrapment of various non-vinylated ligands (such as diphenylcarbazide (DPC), dithizone (DZ), and 8-hydroxyquinoline (8-HQ)) within polymer networks generated by copolymerization of vinylated ligands with a cross-linker [36,37][36][37].
The polymers used to prepare IIPs should contain metal-binding groups. Besides the first work, in which P4-VP was used to prepare IIPs [39], the use of other polymers has also been reported. For instance, Kabanov et al., prepared a copolymer of diethyl vinyl phosphonate and AA by cross-linking with methylenebisacrylamide (MBAAm) in the presence of HMIs [40], and Ohga et al., prepared reusable sorbents by cross-linking metal-complexed CS with chloromethyloxirane for the separation of Cd(II) from Cu(II) and Hg(II) [41]. In the last decade, CS as a functional linear polymer has mainly been used due to its great abundance in nature, non-toxicity, biocompatibility, and biodegradability [33,34][33][34]. Besides CS, the use of ALG and CEL derivatives has also been reported [33].
2.1.3. Cross-Linkers
Cross-linking agents are used to stabilize the entire network. The amount and the nature of the cross-linking agent have a significant impact on the sorption performance of IIPs. Cross-linking agents can be classified into two categories, namely, those comprising vinyl groups that can react with functional monomers (e.g., MBAAm, ethylene glycol dimethacrylate (EGDMA), trimethylolpropane trimethacrylate (TMPTMA), poly(ethylene glycol)diacrylate (PEGDA), and divinylbenzene (DVB)) and those that are only used to cross-link linear or hyper-branched polymers (e.g., glutaraldehyde (GA) and epichlorohydrin (ECH)) [36,37,38][36][37][38]. Insufficient cross-linking agents could lead to IIP with reduced mechanical stability and with randomly distributed recognition sites that can affect the selectivity toward the template ion [38]. On the other hand, the introduction of a high amount of cross-linking agent could generate IIPs with rigid networks, which could reduce the mass transfer performance, chains flexibility, and the number of recognition sites per unit of mass [37,38][37][38].
2.1.4. Initiators
Peroxides and azo compounds are commonly used initiators. The compound azobisisobutyronitrile (AIBN) is widely used as an initiator due to its mild fragmentation conditions. The removal of dissolved oxygen from the polymerization system is a key step because oxygen inhibits free radical polymerization. This can be generally achieved using vacuum extraction, ultrasound, nitrogen, or argon purging [38].
2.1.5. Porogens to Generate Porous 3D Structure within IIPs
In IIP synthesis, the solvent provides the reaction environment for the polymerization reaction. The solvent is responsible for both the dissolution of the reagents required for polymerization (e.g., template ions, functional monomers, cross-linking agents, and initiators) and porosity. The polarity and the dielectric constant are important characteristics of the solvent that directly influence the thermodynamic features of IIPs. These thermodynamic properties do not only affect the porous polymer structures but also play key roles in the preparation of organized/precise imprinting sites. Therefore, the choice of solvent is directly connected to the physicochemical parameters of the template ions, functional monomers, and cross-linking agents. Toluene, methanol, DMF, DMSO, acetonitrile, CHCl3, CH3COOH, and dichloroethane are solvents commonly used [37,38][37][38].
2.1.6. Reagents to Leach out the Template Ion
To leach out the template ion, the most common practice is to wash the IIPs with a strong acid such as HCl, H2SO4, or HNO3 [37]. However, they can exhibit a higher affinity for the ligand than for the target ion. Thus, when some components of the IIP (such as functional monomers) are unstable in acidic conditions, the use of ethylenediaminetetraacetic acid (EDTA) and thiourea as chelating agents is recommended [37].
2.2. Strategies to Prepare IIPs Materials
IIPs can be obtained using multiple preparation methods (Figure 43). The most commonly used approach is free radical polymerization, which includes bulk, precipitation, suspension, and emulsion polymerization methods. The imprinting by polymerization techniques has been deeply described in the publications of Branger et al. [36,37][36][37] and Zhou et al. [38], and thus they will be just briefly presented. To overcome the disadvantages of conventional IIPs, a plethora of technologies have been further promoted such as surface imprinting, stimuli-responsive imprinting, and dual or multiple-component imprinting strategies [38].
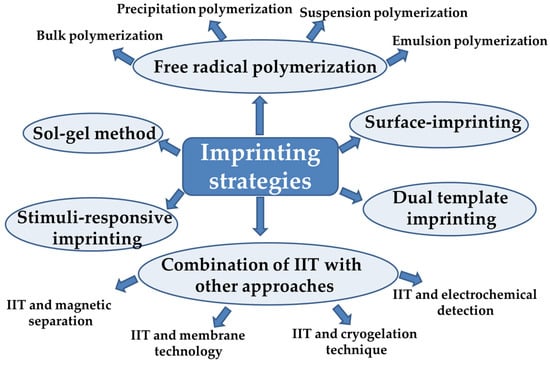
Figure 43.
Strategies applied to prepare IIPs materials (IIT is the abbreviation for ion imprinting technology).
2.2.1. Bulk Polymerization
Bulk polymerization is the earliest and simplest approach to prepare IIPs that do not need special equipment [37,38][37][38]. The template ions, functional monomers, cross-linking agents, and initiators are usually dissolved in good solvents according to an optimum ratio. To obtain IIP particles with a certain dimension, the bulk materials are crushed, ground, and sieved. This procedure can have a major disadvantage since the grinding, crushing, and sieving operations can destroy some binding sites, thus leading to a significant decrease in the yield of the process [37]. In addition, the irregular shape and size of the IIP particles after grounding are an inconvenience for many chromatographic and separation applications. Due to these limitations, many studies have been focused to prepare IIPs directly as beads using homogeneous or heterogeneous polymerization.
2.2.2. Precipitation Polymerization
The reaction mixture consists of a single phase, composed of monomers, ion templates, and initiators, all dissolved in various pore-forming agents. The polymerization is initiated in a homogeneous solution. This technique requires that the obtained polymer be insoluble in the reaction mixture. Once polymerization starts, the first oligomers and insoluble polymer cores start to form [37,38][37][38]. The former remains dissolved in the solvent (porogens), while the nuclei precipitate and continuously grow by adding other monomers and oligomers from the continuous phase [37,38][37][38]. The precipitation polymerization approach is the second most used process after bulk polymerization to prepare IIP in form of particles. This technique allows for good control of the particle dimensions, whereby IIPs in the micrometric or nanometric range can be synthesized by optimizing the monomers to solvent ratio and the stirring of the polymerization mixture. However, one limitation of this method is the use of an excess of porogen, which results in multiple purification steps for its removal. Moreover, the template ion can be easily leached out and some binding sites can be wiped out since fewer cross-linked polymer networks are generated by this technique.
2.2.3. Suspension Polymerization
In suspension polymerization, the synthesis of IIPs occurs in the dispersed phase, which contains template ions, functional monomers, porogens, and initiators that can be regarded as a set of many micro-reactors for carrying out local bulk polymerization [37,38][37][38]. The process requires constant mechanical stirring to keep particles suspended in the continuous phase containing the stabilizers. The suspension polymerization technique typically allows the preparation of IIPs with sizes in the micrometric range (250–550 μm). The porosity of IIPs prepared by suspension polymerization can be adjusted by porogenic solvents. To avoid the passage of the template ion from the dispersed organic phase to the aqueous dispersing phase, the use of inverse suspension polymerization was proposed. In this process, the dispersion phase is a mineral oil [37,38][37][38].
2.2.4. Emulsion Polymerization
For emulsion polymerization, the continuous phase is usually water. In this case, the hydrophobic monomer was dispersed in water using an oil-in-water emulsifier. The continuous phase contains the initiators that produce free radicals [37,38][37][38]. As the reaction proceeds, a growing oil–water interface is generated due to the formation of new polymer cores and their growth. To impede the agglomeration of the growing nuclei, a stabilizing agent is added. Nevertheless, this also represents a drawback since additional purification steps to remove the surfactant from IIP particles are necessary. For this technology, the reverse phase emulsion (water-in-oil) can also be used. With this method the size of the IIPs can be well controlled, allowing for the preparation of uniform microspheres and nanospheres [37,38][37][38].
2.2.5. Sol–Gel Method
Sol–gel method can generate stable and uniform IIPs under mild synthesis conditions. The template ions are introduced into the inorganic network through gel interaction. Organic–inorganic hybrid materials with different shapes, sizes, specific functional moieties, and affinity can be prepared by the sol–gel technique [37,38][37][38]. The IIPs prepared by sol–gel approach exhibit greater thermal and chemical stability than those obtained with conventional imprinting methods [37].
2.2.6. Surface-Imprinting
Conventional imprinting methods for IIPs may result in multiple shortcomings including excessive template ions entrapping depth, difficult leaching, and low regeneration level, poor affinity of the imprinting sites for the target species, reduced mass transfer effect. These drawbacks can be solved by surface-imprinting polymerization technology. The main advantage of the surface-imprinting approach consists in the enhancement of the adsorption efficiency and selectivity through the generation of the recognition sites on the large specific surface area of the carrier [36,37,38][36][37][38]. For surface-imprinting polymerization, identifying carrier materials with stable properties, large specific surface areas, and low price is crucial. In recent years, various IIPs have been fabricated by means of surface-imprinting technology, with supports including activated carbon, silicon materials, magnetic materials, and clay minerals [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38]. Moreover, this technique has been widely investigated to design surface-imprinted materials consisting of various natural polymers with improved selectivity and specificity [33,34][33][34]. However, it should be pointed out that there are some shortcomings in using natural polymers such as the impossibility to include all appropriate functional moieties on natural polymers and their lack of structural stability under harsh conditions [33].
2.2.7. Other Imprinting Technologies
With the development of IIT, strategies based on dual or multiple functional monomers and dual or multiple template ions have attracted increasing attention [36]. As IIT has been developed, many scholars have investigated its combination with other technologies, such as magnetic separation technology, membrane technology, and electrochemical detection technology [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38].
2.3. Evaluation of the Binding Performances of IIPs
Important parameters that influence the sorption performance of IIPs are the chemical nature of ligand groups, the cross-linking density, but also the characteristics of wastewaters, such as the pH and the coexistence of cross-contaminants [36,37,38][36][37][38]. The main challenge when IIPs synthesis is involved is to reach selectivity and enhance the sorption capacity, as well as to obtain an imprinting effect, which is the main proof that the imprinting process was successful. To assess the imprinting effect, a reference polymer must be also prepared and its features should be compared with those of the IIP. The reference material is called non-imprinted polymer (NIP) [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38]. Both the IIP and the corresponding NIP should be studied in the presence of metal ion solutions: either the template ion or a mixture of the template ion and interfering species. This can be performed in batch or flow mode conditions. From the determination of the ion residual concentration in solution after contact with the IIP or NIP, the removal efficiency (RE, %) (Equation (1)) and the adsorption/binding capacity at equilibrium (qe, mg/g) (Equation (2)) are calculated [30,31,32,33,34,35,36,37,38][30][31][32][33][34][35][36][37][38].
where C0 is the initial concentration of metal ions (mg/L), Ce is the concentration of metal ions in the aqueous solution at equilibrium (mg/L), V is the volume of the aqueous solution (L), and m is the mass of IIPs used for extraction (g).
The binding capacity of IIPs materials toward the template ion was also evaluated by applying different mathematical models to the experimental equilibrium and kinetic sorption data (Table 1). Thermodynamic parameters presented in Table 1 have also been calculated by conducting the binding experiments at various temperatures.
Table 1.
Equations of the models commonly used for the theoretical analysis of the sorption data.
| Equation Number | Equation | 1 | Definition |
|---|---|---|---|
| Kinetic models | |||
| (3) | Pseudo-first order (PFO) model | ||
| (4) | Pseudo-second order (PSO) model | ||
| (5) | Intraparticle diffusion (IPD) model | ||
| Isotherm models | |||
| (6) | Langmuir model | ||
| (7) | Freundlich model | ||
| (8) | Dubinin–Radushkevich (D–R) model | ||
| (9) | Sips model | ||
| (10) | Van’t Hoff equation | ||
| (11) | Standard equilibrium constant | ||
| (12) | Standard Gibbs free energy of sorption, kJ mol−1 | ||
1qe and qt—amount of metal ion sorbed at equilibrium and at time t, min, respectively; k1—rate constant of the PFO kinetic model, min−1; k2—rate constant of the PSO kinetic model, g/mg × min; kid—intraparticle diffusion rate constant, mg/g × min1/2; C—constant describing the effect of boundary layer thickness, mg/g; qm—maximum theoretical sorption capacity, mg/g; KL—Langmuir constant, L/mg; KF—Freundlich constant, mg/g × mgN × LN; N—measure of both the nature and strength of adsorption process, and of active sites distribution, related to the surface heterogeneity; the larger is its value, the more heterogeneous the sorbent system is; qDR—maximum sorption capacity of the metal ion, mg/g; KDR—D–R isotherm constant, mol2/kJ2; ε—Polanyi potential; aS—Sips constant; ΔS°—entropy, kJ/mol × K; ΔH°—enthalpy, kJ/mol; R—the gas constant, J/mol × K; T—the temperature in Kelvin; Madsorbate—is the abbreviation of atomic mass of each metal ion.
The ability of the template ion to be recognized by the IIP in the presence of some interfering species, typically other cations, is evaluated by conducting competitive experiments. These can be binary mixtures [37,42][37][42] or multicomponent ones [43]. The selectivity coefficient (k) Equation (13) and the relative selectivity coefficient (k’) Equation (14) have been defined for IIPs by Dai et al. [44], according to the definition given by Kuchen et al., for metal-ion exchange resins [45].
where KD represents the distribution coefficient and is calculated by Equation (15); kIIP and kNIP are the selectivity coefficients of IIP and NIP, respectively.
where qe is the sorption capacity at equilibrium, mg/g; Ce represents the equilibrium concentration, mg/L.
The higher the values of k and k’, the better the imprinting effect.
References
- Hu, H. Human health and heavy metals exposure. In Life Support: The Environment and Human Health; McCally, M., Ed.; MIT Press: Cambridge, UK, 2002; pp. 1–12.
- Can Sener, S.E.; Thomas, V.M.; Hogan, D.E.; Maier, R.M.; Carbajales-Dale, M.; Barton, M.D.; Karanfil, T.; Crittenden, J.C.; Amy, G.L. Recovery of Critical Metals from Aqueous Sources. ACS Sustain. Chem. Eng. 2021, 9, 11616–11634.
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48, 463.
- Saravan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595.
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Tasnim Chowdhury, A.; Rafa, N.; Uddin, A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912.
- Musarurwa, H.; Tawanda Tavengwa, N. Stimuli-responsive polymers and their applications in separation science. React. Funct. Polym. 2022, 175, 105282.
- Ghiorghita, C.-A.; Mihai, M. Recent developments in layer-by-layer assembled systems application in water purification. Chemosphere 2021, 270, 129477.
- Ghiorghita, C.-A.; Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Polysaccharide-Based Composite Hydrogels as Sustainable Materials for Removal of Pollutants from Wastewater. Molecules 2022, 27, 8574.
- Jorge, N.; Santos, C.; Teixeira, A.R.; Marchão, L.; Tavares, P.B.; Lucas, M.S.; Peres, J.A. Treatment of Agro-Industrial Wastewaters by Coagulation-Flocculation-Decantation and Advanced Oxidation Processes—A literature Review. Eng. Proc. 2022, 19, 33.
- Usama Saeed, M.; Hussain, N.; Sumrin, A.; Shahbaz, A.; Noor, S.; Bilal, M.; Aleya, L.; Iqbal, H.M.N. Microbial Bioremediation Strategies with Wastewater Treatment Potentialities—A Review. Sci. Total. Environ. 2022, 818, 151754.
- Farahbakhsh, J.; Vatanpour, V.; Khoshnam, M.; Zargar, M. Recent advancements in the application of new monomers and membrane modification techniques for the fabrication of thin film composite membranes: A review. React. Funct. Polym. 2021, 166, 105015.
- Huang, L.; Liu, R.; Yang, J.; Shuai, Q.; Yuliarto, B.; Kaneti, Y.V.; Yamauchi, Y. Nanoarchitectured porous polymers and their environmental applications for removal of toxic metal ions. Chem. Eng. J. 2021, 408, 127991.
- Cyganowski, P.; Leśniewicz, A.; Polowczyk, I.; Chęcmanowski, J.; Koźlecki, T.; Pohl, P.; Jermakowicz-Bartkowiak, D. Surface-activated anion exchange resins for synthesis and immobilization of gold and palladium nano- and microstructures. React. Funct. Polym. 2018, 124, 90–103.
- Sofińska-Chmiel, W.; Kołodyńska, D. Application of ion exchangers for the purification of galvanic wastewater from heavy metals. Sep. Sci. Technol. 2018, 53, 1097–1106.
- Chen, Y.-G.; Sofinska-Chmiel, W.; Lv, G.-Y.; Kołodynska, D.; Chen, S.-H. Application of Modern Research Methods for the Physicochemical Characterization of Ion Exchangers. Materials 2021, 14, 7067.
- Huang, L.; Shen, R.; Liu, R.; Shuai, Q. Thiol-functionalized magnetic covalent organic frameworks by a cutting strategy for efficient removal of Hg2+ from water. J. Hazard Mater. 2020, 392, 122320.
- Dragan, E.S.; Avram, E.; Dinu, M.V. Organic ion exchangers as beads. Synthesis, characterization and applications. Polym. Adv. Technol. 2006, 17, 571–578.
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Design of porous strong base anion exchangers bearing N,N-dialkyl 2-hydroxyethyl ammonium groups with enhanced retention of Cr(VI) ions from aqueous solution. React. Funct. Polym. 2018, 124, 55–63.
- Alexandratos, S.D. Trends in ion exchange: Analysis of the literature. React. Funct. Polym. 2021, 169, 105066.
- Zaharia, M.-M.; Bucatariu, F.; Vasiliu, A.-L.; Mihai, M. Stable and reusable acrylic ion-exchangers. From HMIs highly polluted tailing pond to safe and clean water. Chemosphere 2022, 304, 135383.
- Bernard, J.; Branger, C.; Beurroies, I.; Denoyel, R.; Blanc, S.; Margaillan, A. Synthesis of a poly(vinylcatechol-co-divinylbenzene) resin and accessibility to catechol units. Polymer 2010, 51, 2472–2478.
- Gendy, E.A.; Ifthikar, J.; Ali, J.; Oyekunle, D.T.; Eikhlifia, Z.; Shahib, I.I.; Khodair, A.I.; Chen, Z. Removal of heavy metals by covalent organic frameworks (COFs): A review on its mechanism and adsorption properties. J. Environ. Chem. Eng. 2021, 9, 105687.
- Dinu, M.V.; Dinu, I.A.; Lazar, M.M.; Dragan, E.S. Insights into the Mechanism of Cu2+ Binding onto Chitosan Based Cryogel Composites: Equilibrium, Kinetics and Thermodynamics Studies. Cellulose Chem. Technol. 2018, 52, 181–192.
- Wu, X. Molecular imprinting for anion recognition in aqueous media. Microchim. Acta 2012, 176, 23–47.
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119.
- Zhang, N.; Zhang, N.; Xu, Y.; Li, Z.; Yan, C.; Mei, K.; Ding, M.; Ding, S.; Guan, P.; Qian, L.; et al. Molecularly Imprinted Materials for Selective Biological Recognition. Macromol. Rapid Commun. 2019, 40, 1900096.
- Mostafa, A.M.; Barton, S.J.; Wren, S.P.; Barker, J. Review on molecularly imprinted polymers with a focus on their application to the analysis of protein biomarkers. Trends Analyt. Chem. 2021, 144, 116431.
- Tchekwagep, P.M.S.; Crapnell, R.D.; Banks, C.E.; Betlem, K.; Rinner, U.; Canfarotta, F.; Lowdon, J.W.; Eersels, K.; van Grinsven, B.; Peeters, M.; et al. A Critical Review on the Use of Molecular Imprinting for Trace Heavy Metal and Micropollutant Detection. Chemosensors 2022, 10, 296.
- Nishide, H.; Deguchi, J.; Tsuchida, E. Selective adsorption of metal ions on crosslinked poly(vinylpyridine) resin prepared with a metal ion as a template. Chem. Lett. 1976, 5, 169–174.
- Rao, T.P.; Kala, R.; Daniel, S. Metal ion-imprinted polymers—Novel materials for selective recognition of inorganics. Anal. Chim. Acta 2006, 578, 105–116.
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Highly selective monitoring of metals by using ion-imprinted polymers. Environ. Sci. Pollut. Res. 2015, 22, 7375–7404.
- Erdem, O.; Saylan, Y.; Andaç, M.; Denizli, A. Molecularly Imprinted Polymers for Removal of Metal Ions: An Alternative Treatment Method. Biomimetics 2018, 3, 38.
- Li, N.; Yang, H. Construction of natural polymeric imprinted materials and their applications in water treatment: A review. J. Hazard. Mater. 2021, 403, 123643.
- Karrat, A.; Lamaoui, A.; Amine, A.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Applications of Chitosan in Molecularly and Ion Imprinted Polymers. Chem. Afr. 2020, 3, 513–533.
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Dano, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083.
- Branger, C.; Meouche, W.; Margaillan, A. Recent advances on ion-imprinted polymers. React. Funct. Polym. 2013, 73, 859–875.
- El Ouardi, Y.; Giove, A.; Laatikainen, M.; Branger, C.; Laatikainen, K. Benefit of ion imprinting technique in solid-phase extraction of heavy metals, special focus on the last decade. J. Env. Chem. Eng. 2021, 9, 106548.
- Zhou, X.; Wang, B.; Wang, R. Insights into ion-imprinted materials for the recovery of metal ions: Preparation, evaluation and application. Sep. Purif. Technol. 2022, 298, 121469.
- Chen, L.; Dai, J.; Hu, B.; Wang, J.; Wu, Y.; Dai, J.; Meng, M.; Li, C.; Yan, Y. Recent progresses on the adsorption and separation of ions by imprinting routes. Sep. Purif. Rev. 2019, 49, 265–293.
- Kabanov, V.A.; Efendiev, A.A.; Orujev, D.D. Complex-forming polymeric sorbents with macromolecular arrangement favorable for ion sorption. J. Appl. Polym. Sci. 1979, 24, 259–267.
- Ohga, K.; Kurauchi, Y.; Yanase, H. Adsorption of Cu2+ or Hg2+ ion on resins prepared by crosslinking metal-complexed chitosans. Bull. Chem. Soc. Jpn. 1987, 60, 444–446.
- Dinu, M.V.; Dinu, I.A.; Lazar, M.M.; Dragan, E.S. Chitosan-Based Ion-Imprinted Cryo-Composites with Excellent Selectivity for Copper Ions. Carbohydr. Polym. 2018, 186, 140–149.
- Humelnicu, D.; Lazar, M.M.; Ignat, M.; Dinu, I.A.; Dragan, E.S.; Dinu, M.V. Removal of Heavy Metal Ions from Multicomponent Aqueous Solutions by Eco-Friendly and Low-Cost Composite Sorbents with Anisotropic Pores. J. Hazard. Mater. 2020, 381, 120980.
- Dai, S.; Burleigh, M.C.; Ju, Y.H.; Gao, H.J.; Lin, J.S.; Pennycook, S.J.; Barnes, C.E.; Xue, Z.L. Hierarchically Imprinted Sorbents for the Separation of Metal Ions. J. Am. Chem. Soc. 2000, 122, 992–993.
- Kuchen, W.; Schram, J. Metal-Ion-Selective Exchange Resins by Matrix Imprint with Methacrylates. Angew. Chem. Int. Ed. Eng. 1988, 27, 1695–1697.
More
