Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 3 by Camila Xu.
Brain-derived neurotrophic factor (BDNF) was named after it was first extracted from porcine brain tissue and identified as a survival factor for neuronal populations that are not responsive to NGF. Beyond its role in neurons, BDNF is also released by keratinocytes, melanocytes, fibroblasts, endothelial cells, platelets, and several immune cells such as T cells, B cells, monocytes, macrophages, mast cells, and eosinophils.
- neurotrophin
- pruritus
- atopic dermatitis
- allergic rhinitis
1. Atopic Dermatitis and BDNF
Previously, it was shown that brain-derived neurotrophic factor (BDNF)-positive eosinophils were located in close vicinity to βIII-Tubulin positive nerve fibers in the skin of patients with atopic dermatitis (AD) [1]. Further, the number of eosinophils found in close proximity to dermal nerve fibers was increased in AD, and BDNF levels in neurons were also elevated. Stimulation of murine dorsal root ganglia (DRG) with BDNF causes outgrowth and branching of nerve fibers [1]. In this regard, it was showed that eosinophils from AD patients expressed functional BDNF by inducing its release with platelet-activating factor (PAF) and subsequently treating DRG neurons with the eosinophil supernatants. The DRG neurons responded with an outgrowth of neurites, demonstrating the ability of eosinophils to structurally modulate sensory nerves [1] (Figure 1). The modulating effect of other immune cells through BDNF on nerve fibers has not yet been described, although a similar effect to that of eosinophils is conceivable. Mast cells are known to be localized in the vicinity of peripheral nerve endings in AD [2], which suggests that neuroimmune interactions might also occur with BDNF. Furthermore, morphological analyses of cutaneous nerves in skin biopsies of AD patients revealed increased numbers of axons in the upper dermis and a significantly higher density of axons in comparison with control subjects [1]. The numbers and length of axons in AD were also higher in lesional skin than in non-lesional skin [3]. This causes a higher density of nerve fibers in the epidermis of AD patients in comparison to control subjects. The increased density is suggested to be associated with functional impairments and involvement in itch sensation. This may be due to hyperinnervation of the skin, which might increase the responsiveness to exogenous trigger factors and endogenous pruritogens [4][5][6]. However, this hypothesis remains controversial since the nerve fiber density does not necessarily correlate with scratching behavior, as shown in a mouse model [7].
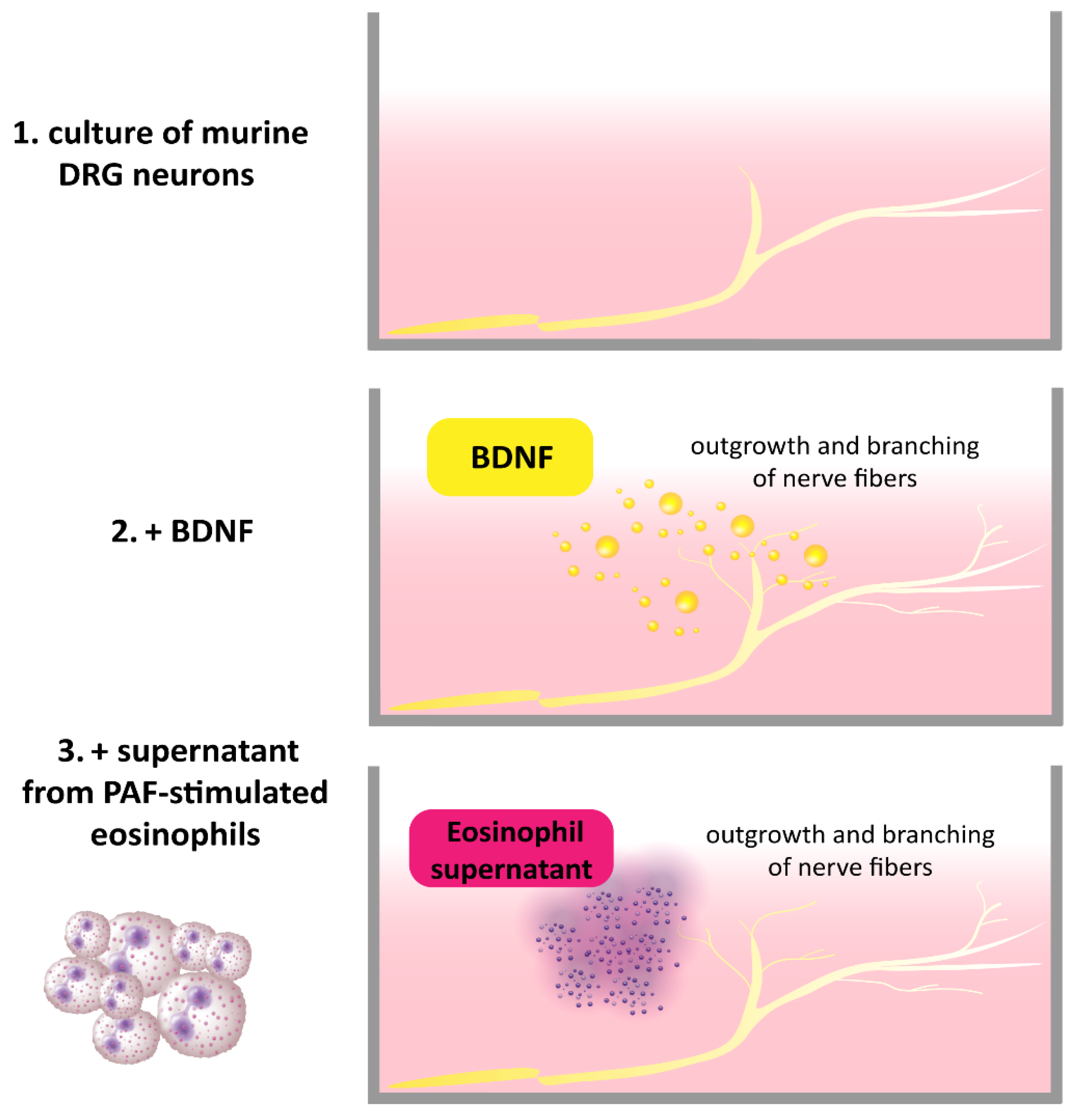
Figure 1. BDNF released by PAF-stimulated AD eosinophils induces the outgrowth and branching of mouse DRG neurons [1].
Furthermore, BDNF might also play a pivotal role in AD, as the plasma, serum, and eosinophils of AD patients contain higher levels of BDNF than those measured in healthy controls [8]. Interestingly, serum levels were also found to correlate with disease severity in adults [9] and children with AD, as well as with scratching behavior [10]. Moreover, increased BNDF levels in AD correlate with the amount of released eosinophil cationic protein (ECP) [10]. Analysis of skin biopsies from AD patients also revealed that eosinophil peroxidase was increased in lesional skin compared to non-lesional and healthy skin [3]. This is interesting, seeing as BDNF has been reported to induce the release of eosinophil peroxidase in eosinophils [11]. However, not only higher expression levels but also findings about the effect of BDNF on eosinophil function provide better insights into its role in AD. It has been shown that BDNF inhibits the apoptosis of eosinophils [8], which in turn might lead to prolonged and amplified total cytokine release and thus could potentially enhance inflammation. Furthermore, BDNF induces chemotactic migration of eosinophils in AD, opposing the effect observed in nonatopic patients [8], which is likely to support eosinophil recruitment into inflamed tissue. However, not only BDNF but also its receptor TrkB, which is expressed in T cells [12], B cells [13], and eosinophils [14], and the low-affinity receptor p75NTR are suggested to contribute to the pathogenesis of AD. Both receptors exhibit higher expression levels in AD than in healthy control subjects [8]. Upregulation of TrkB was also observed in human peripheral blood eosinophils from AD patients [14]. These findings indicate that BDNF is a pivotal player in AD, as it was shown that eosinophils release biologically active BDNF in close vicinity to nerve fibers, modifying their morphology.
2. BDNF in Allergic Rhinitis
BDNF has not only been identified as a crucial factor in skin inflammation like AD but also in AR, which was observed in a study with AR and healthy patients. The expression of BDNF in the nasal mucosa of patients with AR after provocation with allergens was increased when compared to non-allergic subjects [15]. This increase was also positively correlated with the total nasal symptom score in AR after nasal provocation. In contrast, no increased BDNF levels could be measured in the bronchial mucosa after allergen-provocation, though its expression in AR was higher than in healthy patients. The study demonstrated that BDNF serum levels tended to be increased in AR, and allergen provocation further elevated BDNF levels in serum after 24 h of incubation [15]. Interestingly, the BDNF receptor TrkB was downregulated in mast cells after nasal provocation, though patients with AR still exhibited higher expression levels than healthy controls [15]. Levels of BDNF and its receptor might be influenced by other cytokines; however, variants of the BDNF gene have also been identified to have an impact on BDNF release. A single nucleotide polymorphism was found to alter protein function, including the secretion process of BDNF, and was also associated with an increased risk of moderate and severe AR [16]. Besides AR, these genetic variations have also been identified to be associated with a higher susceptibility to allergic asthma [17]. The effect of BDNF on nerve fibers in AR has not been described; however, patients with allergic rhinitis have a greater number of vasoactive intestinal polypeptide (VIP)-positive nerve fibers in the mucosal tissue of the nasal conchae than control subjects. VIP is known for causing vasodilatation and glandular secretion [18]. This suggests higher sensitivity to environmental allergens and more severe symptoms such as sneezing or nasal obstruction. Involvement of BDNF in the morphological changes in AR as observed in the skin seems possible.
3. BDNF in Allergic Asthma
Contrary to the findings in AD, the topic of increased BDNF serum levels in asthma is controversial. While a study from Lommatzsch et al. demonstrated significantly higher serum and plasma levels of BDNF in patients with allergic asthma [19], Joachim et al. did not observe increased serum levels of BDNF in allergic asthma when compared to healthy control subjects [20]. However, BDNF levels in the serum of asthmatic children were found to be inversely correlated with the expiratory volume [21]. Additionally, it has also been observed that BDNF levels in bronchoalveolar lavage fluid (BALF) of asthma patients were elevated after provocation with allergens [22]. These findings might indicate that the release of BDNF in acutely inflamed airways occurs in a more local manner without necessarily increasing systemic levels. BDNF has been described to be expressed by both the bronchial and alveolar epithelium, fibroblasts, the vascular epithelium, and airway smooth muscle cells in human lung tissue [23]. BDNF, derived from human airway smooth muscle cells, has been shown to regulate the release of calcium from the sarcoplasmic reticulum in response to agonists via TrkB. This is suggested to have an impact on the contractility of airway smooth muscle cells in asthma [24]. Another source of high amounts of BDNF are platelets, which were shown to contain reduced amounts of BDNF during respiratory tract infections. This might be due to the enhanced release of BDNF during inflammation [25]. Furthermore, elevated concentrations of BDNF in platelets correlate with bronchial hyperresponsiveness in allergic asthma, and inhibition of BDNF reduces neuronal hyperreactivity to allergens, as shown in an animal model [19]. As observed in the skin, BDNF might change the morphology of nerves in the airways since nerve fiber density was described to be higher in eosinophilic asthma, which was associated with a higher sensitivity to environmental stimuli. It was shown in mice that eosinophils increase epithelial innervation and neuronal reflex bronchoconstriction [26]. Since eosinophils in the skin cause an outgrowth of nerve fibers via BDNF, it might have the same effect on nerves in the airways. All these findings imply that BDNF is also involved in atopic diseases of the respiratory system.
In summary, differing expression levels of BDNF in serum and local fluids of patients with AD and symptom-free subjects indicate that BDNF plays an important role in allergic diseases. This is further substantiated by the fact that allergen provocation also induces varying BDNF levels in AR and allergic asthma. Furthermore, BDNF also promotes morphological changes in neurons in AD as a result of neuroimmune interactions.
References
- Guseva, D.; Rüdrich, U.; Kotnik, N.; Gehring, M.; Patsinakidis, N.; Agelopoulos, K.; Ständer, S.; Homey, B.; Kapp, A.; Gibbs, B.F.; et al. Neuronal branching of sensory neurons is associated with BDNF-positive eosinophils in atopic dermatitis. Clin. Exp. Allergy 2020, 50, 577–584.
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell. Neurosci. 2019, 13, 422.
- Foster, E.L.; Simpson, E.L.; Fredrikson, L.J.; Lee, J.J.; Lee, N.A.; Fryer, A.D.; Jacoby, D.B. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS ONE 2011, 6, e22029.
- Tominaga, M.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212.
- Pereira, M.P.; Mühl, S.; Pogatzki-Zahn, E.M.; Agelopoulos, K.; Ständer, S. Intraepidermal Nerve Fiber Density: Diagnostic and Therapeutic Relevance in the Management of Chronic Pruritus: A Review. Dermatol. Ther. 2016, 6, 509–517.
- Tominaga, M.; Takamori, K. Peripheral itch sensitization in atopic dermatitis. Allergol. Int. 2022, 71, 265–277.
- Kido, M.; Takeuchi, S.; Esaki, H.; Hayashida, S.; Furue, M. Scratching behavior does not necessarily correlate with epidermal nerve fiber sprouting or inflammatory cell infiltration. J. Dermatol. Sci. 2010, 58, 130–135.
- Raap, U.; Goltz, C.; Deneka, N.; Bruder, M.; Renz, H.; Kapp, A.; Wedi, B. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J. Allergy Clin. Immunol. 2005, 115, 1268–1275.
- Raap, U.; Werfel, T.; Goltz, C.; Deneka, N.; Langer, K.; Bruder, M.; Kapp, A.; Schmid-Ott, G.; Wedi, B. Circulating levels of brain-derived neurotrophic factor correlate with disease severity in the intrinsic type of atopic dermatitis. Allergy 2006, 61, 1416–1418.
- Fölster-Holst, R.; Papakonstantinou, E.; Rüdrich, U.; Buchner, M.; Pite, H.; Gehring, M.; Kapp, A.; Weidinger, S.; Raap, U. Childhood atopic dermatitis-Brain-derived neurotrophic factor correlates with serum eosinophil cationic protein and disease severity. Allergy 2016, 71, 1062–1065.
- Noga, O.; Englmann, C.; Hanf, G.; Grützkau, A.; Guhl, S.; Kunkel, G. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin. Exp. Allergy 2002, 32, 1348–1354.
- Polakowski, N.; Terol, M.; Hoang, K.; Nash, I.; Laverdure, S.; Gazon, H.; Belrose, G.; Mesnard, J.-M.; Césaire, R.; Péloponèse, J.-M.; et al. HBZ stimulates brain-derived neurotrophic factor/TrkB autocrine/paracrine signaling to promote survival of human T-cell leukemia virus type 1-Infected T cells. J. Virol. 2014, 88, 13482–13494.
- D’Onofrio, M.; de Grazia, U.; Morrone, S.; Cuomo, L.; Spinsanti, P.; Frati, L.; Gulino, A.; Ragona, G. Expression of neurotrophin receptors in normal and malignant B lymphocytes. Eur. Cytokine Netw. 2000, 11, 283–291.
- Raap, U.; Deneka, N.; Bruder, M.; Kapp, A.; Wedi, B. Differential up-regulation of neurotrophin receptors and functional activity of neurotrophins on peripheral blood eosinophils of patients with allergic rhinitis, atopic dermatitis and nonatopic subjects. Clin. Exp. Allergy 2008, 38, 1493–1498.
- Raap, U.; Fokkens, W.; Bruder, M.; Hoogsteden, H.; Kapp, A.; Braunstahl, G.-J. Modulation of neurotrophin and neurotrophin receptor expression in nasal mucosa after nasal allergen provocation in allergic rhinitis. Allergy 2008, 63, 468–475.
- Jin, P.; Andiappan, A.K.; Quek, J.M.; Lee, B.; Au, B.; Sio, Y.Y.; Irwanto, A.; Schurmann, C.; Grabe, H.J.; Suri, B.K.; et al. A functional brain-derived neurotrophic factor (BDNF) gene variant increases the risk of moderate-to-severe allergic rhinitis. J. Allergy Clin. Immunol. 2015, 135, 1486–1493.e8.
- Andiappan, A.K.; Parate, P.N.; Anantharaman, R.; Suri, B.K.; Wang, D.Y.; Chew, F.T. Genetic variation in BDNF is associated with allergic asthma and allergic rhinitis in an ethnic Chinese population in Singapore. Cytokine 2011, 56, 218–223.
- Fischer, A.; Wussow, A.; Cryer, A.; Schmeck, B.; Noga, O.; Zweng, M.; Peiser, C.; Dinh, Q.T.; Heppt, W.; Groneberg, D.A. Neuronal plasticity in persistent perennial allergic rhinitis. J. Occup. Environ. Med. 2005, 47, 20–25.
- Lommatzsch, M.; Schloetcke, K.; Klotz, J.; Schuhbaeck, K.; Zingler, D.; Zingler, C.; Schulte-Herbrüggen, O.; Gill, H.; Schuff-Werner, P.; Virchow, J.C. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 115–120.
- Joachim, R.A.; Noga, O.; Sagach, V.; Hanf, G.; Fliege, H.; Kocalevent, R.D.; Peters, E.M.; Klapp, B.F. Correlation between immune and neuronal parameters and stress perception in allergic asthmatics. Clin. Exp. Allergy 2008, 38, 283–290.
- Szczepankiewicz, A.; Rachel, M.; Sobkowiak, P.; Kycler, Z.; Wojsyk-Banaszak, I.; Schöneich, N.; Skibinska, M.; Bręborowicz, A. Serum neurotrophin-3 and neurotrophin-4 levels are associated with asthma severity in children. Eur. Respir. J. 2012, 39, 1035–1037.
- Virchow, J.C.; Julius, P.; Lommatzsch, M.; Luttmann, W.; Renz, H.; Braun, A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am. J. Respir. Crit. Care Med. 1998, 158, 2002–2005.
- Prakash, Y.S.; Martin, R.J. Brain-derived neurotrophic factor in the airways. Pharmacol. Ther. 2014, 143, 74–86.
- Prakash, Y.S.; Thompson, M.A.; Pabelick, C.M. Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 2009, 41, 603–611.
- Lommatzsch, M.; Niewerth, A.; Klotz, J.; Schulte-Herbrüggen, O.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. Platelet and plasma BDNF in lower respiratory tract infections of the adult. Respir. Med. 2007, 101, 1493–1499.
- Drake, M.G.; Scott, G.D.; Blum, E.D.; Lebold, K.M.; Nie, Z.; Lee, J.J.; Fryer, A.D.; Costello, R.W.; Jacoby, D.B. Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci. Transl. Med. 2018, 10, eaar8477.
More
