1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the causative agent of Coronavirus Disease 2019 (COVID-19), rapidly reached pandemic proportions resulting in more than 600 million confirmed cases in all age groups around the world
[1]. In the United States of America (USA), there have been more than 14 million COVID-19 cases, according to the Centre for Disease Control and Prevention (CDC)
[2]. Although COVID-19 is usually mild in the pediatric population, some children rarely present with severe clinical manifestations. In April 2020, the first reports from the United Kingdom described a clinical presentation in children that shares similar clinical and laboratory characteristics with incomplete Kawasaki Disease (KD) or Toxic Shock Syndrome (TSS)
[3]. The condition has been called Multisystem Inflammatory Syndrome in Children (MIS-C), associated with SARS-CoV-2 infection, and there have been many cases documented worldwide
[4][5][6][4,5,6]. The same condition is encountered in the literature as Pediatric Multisystem Inflammatory Syndrome (PMIS), Pediatric Inflammatory Multisystem Syndrome temporally associated with SARS-CoV-2 (PIMS-TS), pediatric hyperinflammatory syndrome or pediatric hyperinflammatory shock
[7].
MIS-C represents a relatively small proportion of the total COVID-19 cases in children. According to the CDC, as of January 2023, there are over 9300 total cases with a median age of 9 years that meet the criteria of MIS-C and 76 reported deaths (estimated mortality of 0.8%)
[8]. Compared to the predominance periods of the Alpha, Beta and Delta variants of SARS-CoV-2, it is evident that MIS-C is significantly less frequent and less severe during the Omicron period
[9]. The CDC and the World Health Organization (WHO) case definitions of MIS-C have some differences. Both definitions require the presence of fever, elevated inflammatory markers, at least two organ system involvement, evidence of SARS-CoV-2 infection or exposure, and the exclusion of other potential causes
[10][11][10,11]. The definitions mainly differ in the duration of the fever, as well as hospitalization requirements. The CDC case definition requires that the child must have severe symptoms requiring hospitalization, whereas the WHO case definition does not
[10][11][10,11].
Even though the timing of symptoms onset in COVID-19 varies per case, the usual MIS-C onset is between 2–6 weeks after acute infection, but rare cases of MIS-C occurring > six weeks after the acute SARS-CoV-2 infection have also been reported
[12]. In some cases, the time interval between acute infection and the onset of MIS-C symptoms remains unknown due to asymptomatic acute infection. Interestingly, MIS-C cases seem to increase at least 3–6 weeks after a peak of SARS-CoV-2 transmission within society
[13][14][13,14]. This time interval coincides with the time of development of acquired immunity and suggests the postinfectious nature of MIS-C.
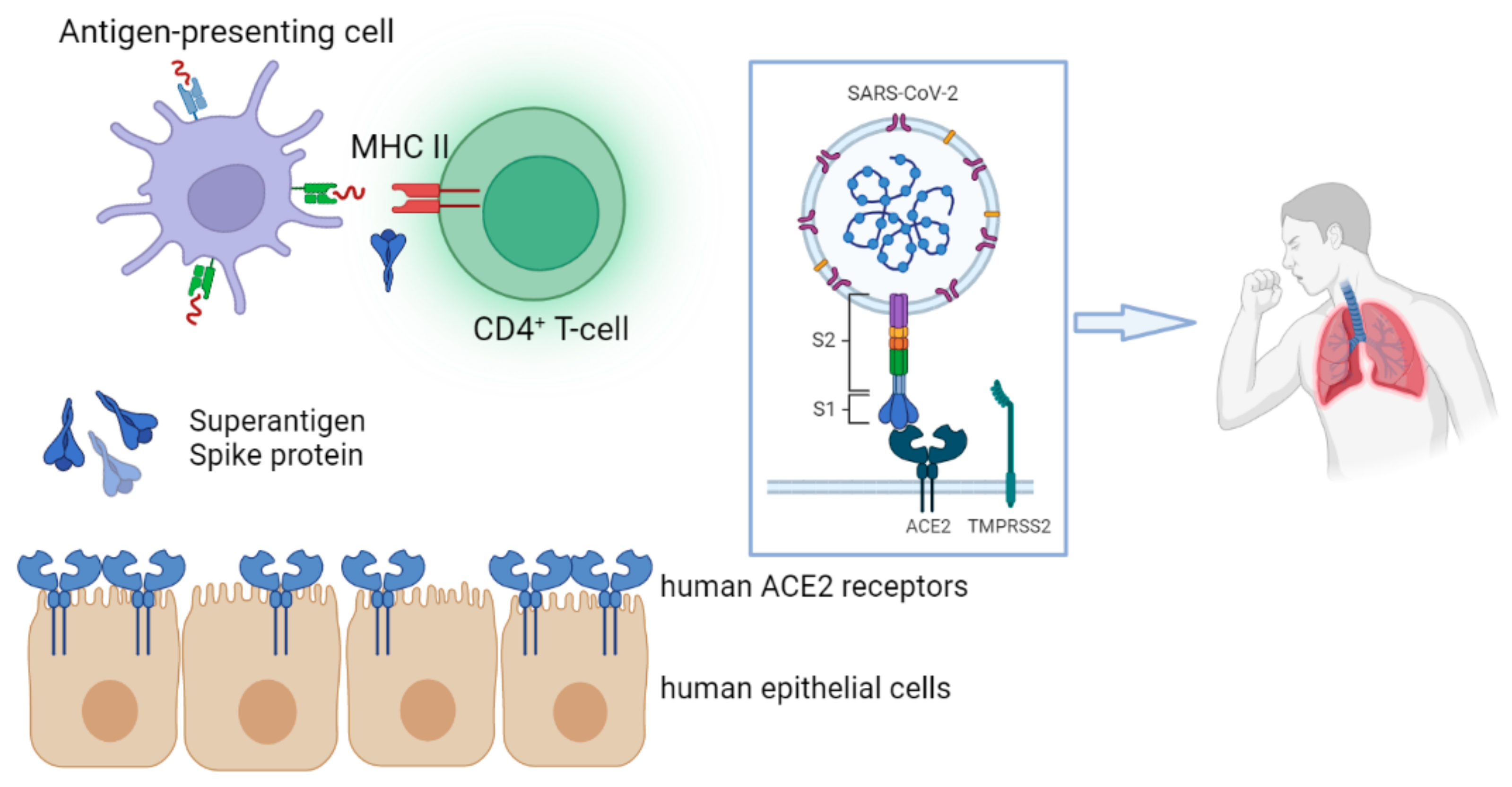
The pathophysiology of MIS-C is not fully recognized. It is indicated that MIS-C is the result of an abnormal immune dysregulation of the virus comparable to KD, macrophage activation syndrome (MAS), and cytokine release syndrome. However, it appears to have an immunophenotype that is distinct from KD and MAS
[15][16][15,16].
In this rentryview, the innate and adaptive immunological aspects of MIS-C are discussed based on current evidence, and the great heterogeneity between SARS-CoV-2 immunological studies and the genetic background of patients with MIS-C are discussed.
2. Incidence of MIS-C
Nowadays, MIS-C has a worldwide distribution, but there are notable differences among different world regions and different SARS-CoV-2 variants. As by the end of February 2023 in the USA, the highest incidence of MIS-C in 50 different jurisdictions was encountered in California, followed by Texas, Florida, Georgia, Ohio and New York, while in other states, such as Oklahoma or Nevada, reported cases were relatively lower
[8].
Data from the USA, Europe, Asia and Africa indicate a lower incidence of MIS-C during Delta and Omicron variant predominance periods. In the USA, MIS-C incidence and complications were significantly reduced during the Delta period compared to previous pandemic waves
[17]. The same trend was also confirmed in Greece, with the estimated incidence of MIS-C being notably reduced from 3.5/1000 in Alpha to 0.25/1000 in the Delta predominance period
[18]. After the onset of Omicron, the incidence and severity of MIS-C was even lower compared to Alpha and Delta variant waves in Israel
[19], while in a study in South Africa in November 2021, no patients who fulfilled WHO criteria for MIS-C diagnosis were encountered
[9]. It is possible that MIS-C epidemiology has remarkably changed as a consequence of widespread exposure of the pediatric population to successive COVID-19 waves, particularly the Omicron variant, mainly characterized by increased transmissibility or reinfection rates.
Pre-existing SARS-CoV-2 immunity, either as a result of natural infection or vaccination in children, seems to play a crucial role in MIS-C pathogenesis and severity. According to CDC, SARS-CoV-2 seroprevalence attributed only to infection-induced immunity has reached 92% in children by March 2023, while a significant increase in seropositivity during Omicron compared to Delta variant in the pediatric population has also been observed in Europe as well
[20]. However, SARS-CoV-2 vaccination has also affected MIS-C incidence. During the Omicron wave, a study by Holm et al. showed that the incidence of MIS-C was considerably reduced among vaccinated children in contrast with individuals who had not been vaccinated (one vaccinated vs. 11 unvaccinated MIS-C patients)
[21].
3. Innate Immune Responses in MIS-C
Overexpression of certain innate immunological pathways might lead not only to MIS-C onset but also to increased MIS-C severity and complications. These pathways do involve not only innate immune system cells and inflammatory markers but also cytokines, chemokines and certain gene activation.
MIS-C is a severe condition with abnormal blood cell counts, elevated inflammatory markers, and pro-inflammatory mediators disturbances, some of the most common characteristics during the onset of the syndrome
[15]. Lymphopenia, neutrophilia, and elevated inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), D-dimers, procalcitonin, fibrinogen and ferritin are detected in most patients with MIS-C and correlate with disease severity
[22][23][22,23]. Among patients with MIS-C, a higher mean CRP value (32.1 mg/dL, normal values: 0–0.5 mg/dL) was observed in those who developed shock, as was an increased neutrophil count (16 × 10
9/L, normal values: 1.5–7 × 10
9/L) and a decrease in lymphocyte count (0.7 to 1.3 × 10
9/L, normal values: 1.5–4 × 10
9/L) in those who did not
[22]. Thrombocytopenia and low eosinophil counts are the main distinguishing characteristics of MIS-C compared to KD and appear to be related to the degree of viral pandemic (ViP) signatures activation, the levels of IL-15 and MIP1 expression, which is a critical component of the immune system component
[24]. Compared to Kawasaki, patients with MIS-C have significantly lower absolute neutrophil, lymphocyte, platelet, and eosinophil values
[24].
Neutrophils, the most abundant circulating phagocytes, are stimulated by several major chemoattractant factors, such as complement-derived C5a and the neutrophil chemokine interleukin-8 (IL-8)
[25][55]. In pediatric patients with COVID-19, there is interferon-mediated gene stimulation in neutrophils
[26]. In a study by Seery et al., a negative association of neutrophils with CD11b, CD66b, and L-selectin was observed, while CD64 in neutrophils was a marker of disease severity as it varied significantly between asymptomatic and symptomatic COVID-19 children
[27][25]. The expression of CD11b, CD66b, LAIR-1, and PD-L1 in neutrophils is significantly higher in MIS-C patients
[27][25]. In MIS-C, there is a notable up-regulation of the
CCRL2 ELMO2,
GPR84,
IRF7,
IFIT3, and
MX1 genes, which are responsible for neutrophil chemotaxis and are enrolled in multiple immunological pathways, including type I and II interferon activation or β2-integrin stimulation
[26]. However, the expression of antigen-presenting cells is relatively reduced
[28][27]. It is possible that MIS-C patients may be characterized by neutrophil extracellular traps (NET), a condition commonly encountered in adults with moderate and severe COVID-19 associated with thrombosis and endothelial damage
[29][30][28,29]. Seery et al. showed that there are no significant differences between children with COVID-19 and MIS-C in NETs
[27][25]. Further studies in the field of NETs are required in order for a pathophysiological connection with MIS-C to be revealed.
The role of NK cells in the pathogenesis of MIS-C remains elusive. Current evidence supports the increased expression of genes, such as
CCL4, which are associated with increased cytotoxic potential and impairment in peripheral tissues
[28][27]. There is a positive correlation between IFN-gamma levels in plasma with NCR1, the soluble marker of NK cells
[31].
In a study by de Cevins et al., NF-kB signaling, VEGF signaling, and inflammatory pathways (type I and type II IFNs, IL-1, IL-6, and IL-17 signaling pathways) were substantially outnumbered in patients with MIS-C
[32][30]. A sampling of monocytes and dendritic cells from MIS-C patients revealed a significant decrease in the expression of NF-kB inhibitors, including NFKBIA, NFKBID, NFKBIE, and NFKBIZ. In MIS-C without myocarditis, the expression of TRAIL, IL-7, IL-2, IL-13, IFN-g, IFN-a2, IL-17A, and Granzyme B was slightly enhanced compared to MIS-C with myocardial involvement
[32][30]. A modification of type I and type II IFN signaling pathways, alongside overexpression of many IFN-stimulated genes (ISGs) (
JAK2, STAT1, STAT2, IFITM1, IFITM2, IFI35, IFIT1, IFIT3, MX1, IRF1) in dendritic cells and monocytes was also shown in patients with MIS-C without myocarditis exclusively
[32][30]. Increasing VEGF, TGF-a, and TGF-b may constitute potential mediators of angiogenesis and vascular homeostasis, while elevated chemokines (CCL2, CCL3, CCL20, CX3CL1, and CX10; CCL2, CCL3, CCL20, CX3CL1, and CX10, respectively) may facilitate enhanced cell migration toward inflamed sites
[32][30]. Inflammatory cytokines, such as TNF-a, TGF-b, IL1b, IL-13, IL-4, and VEGF, are possible to enhance cardiac fibroblasts’ development into cardiac myofibroblasts
[32][30].
Cytokines and chemokines play a vital role in the initiation, prolongation or downregulation of the immune response in the COVID-19 in the pediatric population. In MIS-C, certain cytokines, such as TNF, IL-1β, IFN-gamma, IL-6, IL-8, IL-10 and IL-17 and chemokines, such as CCL2, CCL3, CCL4, CXCL1, CXCL5, CXCL6, CXCL9, CXCL10 or CXCL11, can be elevated compared to pediatric patients with uncomplicated COVID-19
[15][16][28][33][34][35][36][15,16,27,32,33,34,35]. Plasma IL-6 was elevated in patients with severe MIS-C, but levels were not superior when compared to IL-6 levels in pediatric septic patients
[37][56]. Apart from IL-1 and IL-8, which were not significantly elevated in MIS-C compared to KD, there is a robust enhancement of TNF-a, IFN-gamma and IL-10 production in MIS-C compared to KD (
Table 12)
[24]. The inflammatory mediator IL-17 plays a more prominent role in the progress of KD than in MIS-C (
Table 12)
[38][53], while cytokine profiles demonstrated that patients with severe MIS-C had higher levels of circulating IFN compared to those with KD (
Table 12)
[39][54]. It is important to state that the levels of cytokines and chemokines may significantly vary among studies that use different proportions of age groups, the timing of sampling or diagnostic methods. Patients with potentially fatal COVID-19 were found to carry uncommon mutations in at least 13 loci that result in compromised IFN pathways, according to research by Zhang et al.
[40][57].
Table 1. Innate immunological differences encountered in MIS-C compared to Kawasaki disease.
| Immunological Factors |
MIS-C |
Kawasaki Disease |
References |
| Absolute neutrophil counts |
↓ |
↑ |
[24] |
| Absolute lymphocyte counts |
↓ |
↑ |
[24] |
| Absolute platelet counts |
↓ |
↑ |
[24] |
| Absolute eosinophil counts |
↓ |
↑ |
[24] |
| IFN-gamma CXCL9 values |
↑ |
↓ |
[38] |
| IL-1 and IL-8 |
↑ |
↑ |
[24] |
| TNF-a, IFN-gamma and IL-10 |
↑ |
↑ |
[24][39] |
| IL-17 |
↑ |
↑ |
[38] |