You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Sabrin R. M. Ibrahim and Version 2 by Sirius Huang.
Fungi serve as a depository of fascinating, structurally unique metabolites with considerable therapeutic significance. Aspergillus genus represents one of the most prolific genera of filamentous fungi. Aspergillus nidulans Winter G. is a well-known and plentiful source of bioactive metabolites with abundant structural diversity, including terpenoids, benzophenones, sterols, alkaloids, xanthones, and polyketides, many of which display various bioactivities, such as cytotoxicity, antioxidant, anti-inflammatory, antiviral, and antimicrobial activities.
- fungi
- Aspergillus nidulans
- Trichocomaceae
- metabolites
- biosynthesis
1. Introduction
Several studies reported the separation and characterization of assorted classes of metabolites, employing various chromatographic and spectral tools that are surveyed herein, along with their estimated bioactivities and postulated biosynthesis pathways. It was noted that among the diverse metabolites reported from this species, a limited number of them have been assessed for biological capacity.
2. Benzophenones
A. nidulans and its teleomorph Emericella nidulans are known to biosynthesize prenylated structurally related benzophenones [1][2][3][43,44,45]. Many investigations remarked upon the separation of arugosins, which are interesting fungal metabolites that involve the biosynthesis of structurally related polyketides (e.g., anthraquinones, anthrones, xanthones) [1][2][3][4][5][31,43,44,45,46].
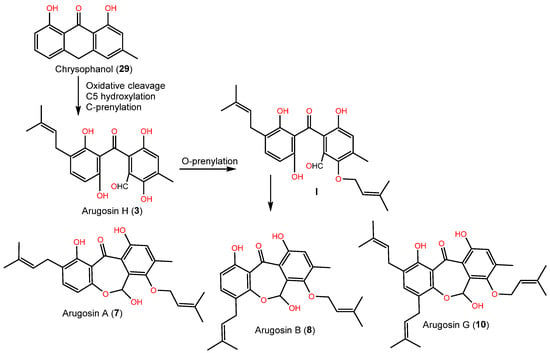
The fungus E. nidulans var. acristata, isolated from a Mediterranean green alga, yielded two new benzophenones, arugosins G (10) and H (3), together with arugosins A (7) and B (8) that were separated from the EtOAc extract by Sephadex LH-20/HPLC (Figure 1). These metabolites featured prenyl moieties that are connected to the skeleton via an ether linkage. Compound 3 had antifungal and antialgal potential (IZDs 2 and 3 mm, respectively) vs. Mycotypha microspora and Chlorella fusca, respectively, whereas 7 and 8 (mixture) revealed antibacterial influence vs. Bacillus megaterium (IZD 4 mm) in the agar diffusion assay [1][43]. Additionally, 7 and 8 demonstrated modest antitumor effectiveness vs. individual cancer cell lines [1][43]. It is noteworthy that arugosins A (7) and B (8), dibenz[b,e]oxepin metabolites that were formerly reported from A. silvaticus, A. rugulosus, and A. variecolor, and arugosins C and F were obtained from Aspergillus spp. and Ascodesmis sphaerospora, respectively [1][43].
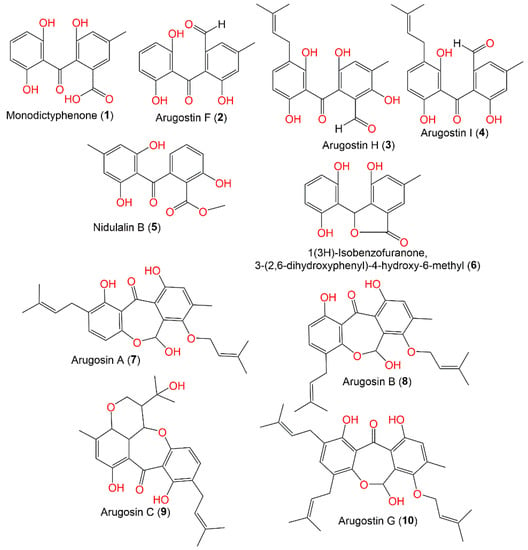
Figure 1.
Structures of benzophenones (
1
–
10
) reported from
Aspergillus nidulans
.
Arugosin H (3) was proposed as originating from chrysophanol (29, anthrone), which performs oxidative cleavage to produce the aldehyde group, which subsequently undergoes hydroxylation and C-prenylation (Scheme 1). Further, the aldehyde group is converted to a hemiacetal function to produce tricyclic and prenylated metabolites 7, 8, and 10 [1][43].
3. Xanthones and Quinones
Xanthones are dibenzo-γ-pyrone derivatives that differ in the substituents on the benzene rings, relying on modification reactions and biosynthetic origins. Fungal xanthones are polyketides created mainly from acetate units and commonly contain a CH3 group. It was reported that A. nidulans produces prenylated xanthones with varied structures.
Compounds 14, 16, and 18 are xanthones reported from green alga-derived E. nidulans var. acristata. Among them, 18 had antifungal and antialgal capacity (IZDs 11 and 5 mm, respectively) vs. M. microspora and C. fusca. However, none of them exhibited immune-stimulatory potential against cytokine induction in PBMCs [1][43]. Compounds 14 and 18 were reported from A. nidulans and isolated from Red Sea brown alga, Turbinaria elatensis, using SiO2 and Sephadex LH-20 CC. They had mild inhibition capacity on HCV NS3/4A protease (IC50s 48.5 and 50.0 μg/mL, respectively), compared to HCV-12 (IC50 1.5 μg/mL) [6][47]. In 2018, isosecosterigmatocystin (15), a new xanthone, in addition to 18, was separated and identified from the EtOAc culture extract of A. nidulans MA-143 under 0.1% ethanol stress. Compound 15 was assigned as having 2′S/3′S based on X-ray analysis [7][30].
The cytotoxic capacity of 18 and 20 vs. NCI-H460, MCF-7, and HeLa cell lines in the SRB was assessed. Only 18 (IC50 50 μM/mL) possessed growth inhibition capacity vs. the MCF-7 cell, whereas 20 was inactive (IC50 > 100) [8][53] (Figure 2).
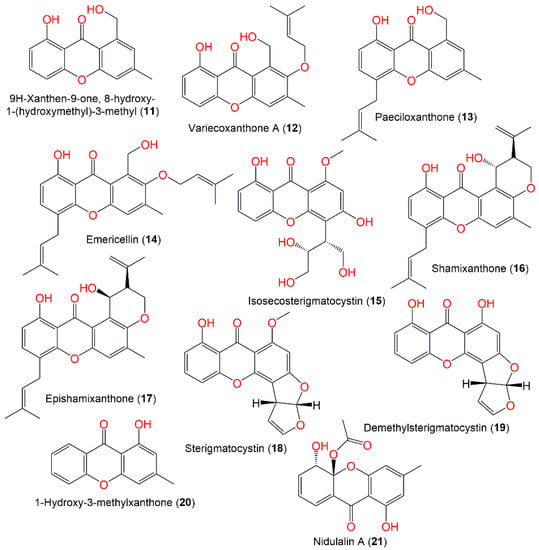
Figure 2.
Structures of xanthones (
11
–
21
) reported from
Aspergillus nidulans
.
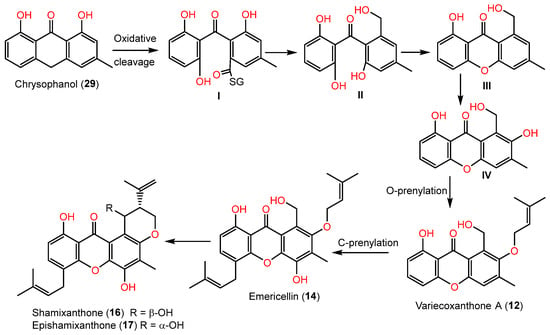
Also, the xanthones were proposed to be biosynthesized from 29, which is transformed by quinone ring oxidative cleavage into thiolester I. Its oxidoreductase-catalyzed reduction yields benzophenone alcohol (II). The alcohol (II) produces 1-hydroxy-6-methyl-8- hydroxymethylxanthone (III) via H2O elimination, and then hydroxylation via monooxygenase produces 1,7-dihydroxy-6-methyl-8-hydroxymethylxanthone (IV). The latter’s prenyltransferase-catalyzed prenylation results in 12, which is C-prenylated to form 14. Subsequent cyclization of 14 produces 16 and 17 (Scheme 2) [3][45].
Nidurufin (42), a new metabolite of which is hydroxyaverufin, and 39 were purified from the CHCl3:MeOH extract of A. nidulans G-106 using silica and formamide-impregnated cellulose powder and characterized by spectral and chemical methods (Figure 3) [9][51].
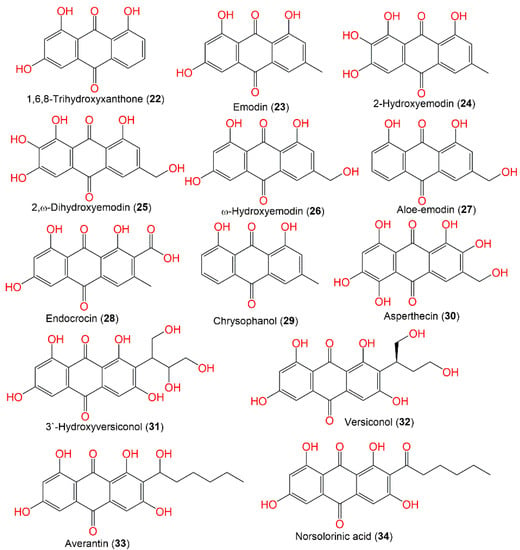
Figure 3.
Structures of quinones (
22
–
34
).
The aggregation of the microtubule-accompanied protein tau is substantial in various neurodegenerative illnesses, such as Alzheimer’s disease. Interestingly, 25 and 30 demonstrated potent tau aggregation inhibition potential, which was observed in a filter trap assay and by electron microscopy. In addition, 25 and 30 lessened tau aggregation without blocking tau stabilization of microtubules, like 23.
Together, these metabolites demonstrated potential as lead compounds for developing tau aggregation inhibitors in Alzheimer’s disease and neurodegenerative tauopathies [10][54]. Isoversicolorin C (36), a new anthraquinone derivative, and six related metabolites—34, 35, 39, 45, and 44—were purified and characterized from the EtOAC culture extract of mangrove-derived A. nidulans MA-143 under ethanol 0.1% stress (Figure 4). Their isolation and elucidation were accomplished by SiO2/RP-18/Sephadex LH-20 and NMR/ECD/X-ray. Compound 36 had potent antibacterial capacity vs. E. ictaluri and V. alginolyticus (MICs 4.0 and 1.0 μg/mL, respectively); however, 35 possessed capacity vs. M. luteus, E. coli, V. parahaemolyticus, V. alginolyticus, and E. ictaluri (MICs ranged from 1.0 to 8.0 μg/mL), which were comparable to chloramphenicol [7][30].
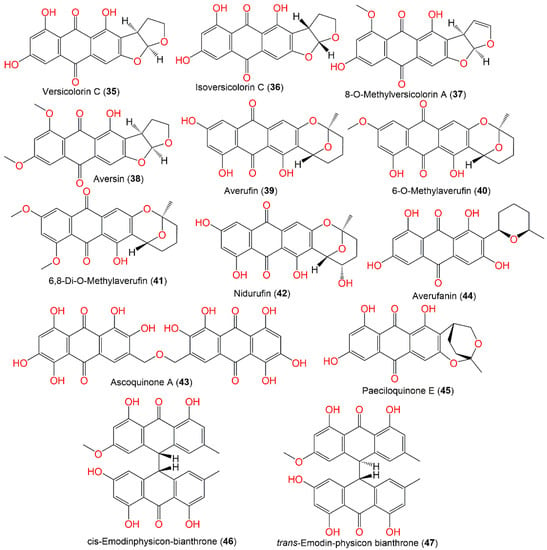
Figure 4.
Structures of quinones (
35
–
47
).
4. Depsidones and Biphenyl Ethers
Depsidones consist of two 2,4-dihydroxy-benzoic acid moieties, connecting via ester and ether bonds, resulting in 11H-dibenzo[b,e] [11][12][1,4] dioxepin-11-one core formation [13][59]. They originated from depsides’ direct-oxidative coupling of the A 2OH ring and the B 5′ carbon ring. These metabolites were found to have diversified potential: anti-malarial, herbicidal, anti-leishmanial, larvicidal, antifungal, cholinesterase and aromatase inhibitory, and antioxidant activity [13][59].
Compounds 48 and 49 were separated from the A. nidulans mycelia’s light petroleum ether extract by repeated crystallization from the EtOH and petroleum ether. Compound 48 is a monomethyl ether that completely suppressed Mycobacterium tuberculosis growth for 4 weeks, as well as Microsporum audouini and Trichophyton tonsurans, and was inactive against bacteriophages [14][60].
The co-culture of A. nidulans and A. fumigatus enhanced the production of diphenyl ethers, 52–55, which were separated by SiO2 CC/HPLC and identified by NMR tools (Figure 5). These metabolites (52–55) possessed antibacterial potential against B. subtilis (MICs 6.3–100 μg/mL) compared to chloramphenicol, indicating that the hydroxylation enhanced the antibacterial effect of diphenyl ethers [15][32]. In addition, 56 revealed potent antimicrobial effectiveness (MICs 2–4 μg/mL) vs. E. ictarda, A. hydrophilia, E. tarda, and V. harveyi (aquatic pathogens) and C. gloeosporioides (pathogenic plant fungus), which could be further studied in terms of developing antimicrobial agents [4][31].
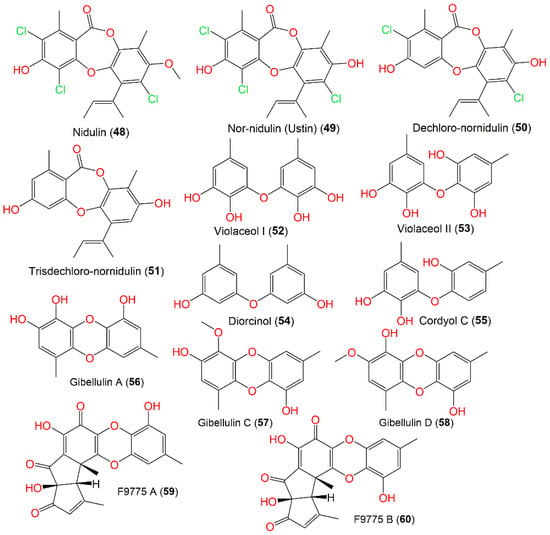
Figure 5.
Structures of depsidones (
48
–
51
) and biphenyl ethers (
52
–
60
).
5. Alkaloids
5.1. Quinolone Alkaloids
Natural quinoline alkaloids are an abundant class of alkaloids that have been reported as having animal, plant, and microbial origins. Some investigations remarked upon the separation of quinoline alkaloids from A. nidulans.
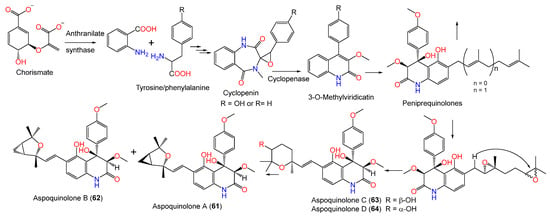
A combined analytical and genomic approach and metabolic investigation of A. nidulans HKI 0410 under various culture conditions resulted in aspoquinolones A-D (61–64), which were separated utilizing SiO2/Sephadex LH-20/HPLC and assigned by NMR [16][62]. Compounds 61 and 62 are isomers with 3-methoxy-4,6-dihydroxy-4-(4′-methoxyphenyl)quinolinone and unusual 2,2,4-trimethyl-3-oxa-bicyclo[3.1.0]hexane moieties; however, 63 and 64 are diastereomers (ratio 2:1) sharing the same 3-methoxy-4,6-dihydroxy-4-(4′-methoxyphenyl)quinolinone skeleton as 61 and 62 (Figure 6). Compounds 61 and 62 exhibited remarkable cytotoxic and antiproliferative capacity on L-929 (GI50 10.6/11.4 μg/mL) and K-562 (GI50 17.8/21.2 μg/mL) cell lines, respectively.
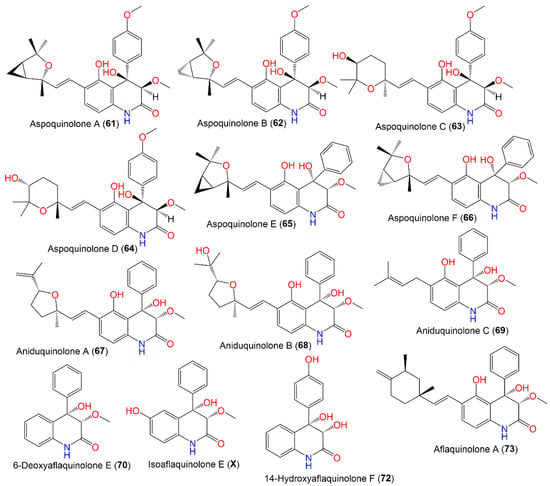
Figure 6.
Structures of quinoline alkaloids (
61
–
73
).
These metabolites were postulated as originating from cyclopenin (benzodiazepine, I) formed from anthranilic acid and tyrosine/or phenylalanine, which is biosynthesized by anthranilate synthase (Scheme 3). This enzyme catalyzed the conversion of chorismate to anthranilic acid for tryptophan biosynthesis [16][62].
From Annelida Whitmania pigra-associated A. nidulansi, aspoquinolones E (65) and F (66), new prenylated quinolinone alkaloids were purified utilizing SiO2/Sephadex LH-20/HPLC. Their configurations and structures were determined based on spectral and ECD analyses. They featured 3S/4S configuration, with an unusual 2,2,4-trimethyl-3oxa-bicyclo[3.1.0]hexane moiety. Their cytotoxic evaluation vs. A-549, HL-60, and MCF-7 revealed that 65 exhibited moderate cytotoxic capacity (IC50s 3.50, 29.15, and 24.5 μM, respectively) compared to cis-platin (IC50s 13.17, 3.22, and 22.96 μM, respectively). It is noteworthy that 65 and 66, with the same structure but different cyclopropyl ring configuration, were greatly varied in activity, suggesting that the cyclopropyl ring configuration influenced the activity [17][33].
In 2013, the separation of new 4-phenyl-3,4-dihydroquinolone derivatives, 67–72, along with known related analog 73, was reported from a Rhizophora stylosa-associated A. nidulans MA-143 extract by SiO2/RP-18/sephadex LH-20/HPLC. Their structures and absolute configuration were established by spectral, ECD, and X-ray analyses. Compounds 70–72 share with 67–69 the 4-phenyl-3,4-dihyroquinolone moiety but lack the terpenoid side chain, as in 67–69. Additionally, 67 was related to 73; however, 67 lacks the cyclohexane moiety in the terpenoid side chain of 73; instead, it features a terminal double bond and substituted tetrahydrofuran moiety. These metabolites did not have cytotoxic (vs. BEL-7402, MDA-MB-231, HL-60, and K562) or antibacterial (E. coli and S. aureus) potential in the MTT and well diffusion methods, respectively, while 68, 69, and 73 had a marked brine shrimp lethality (LD50 7.1, 4.5, and 5.5 μM, respectively) that was more powerful than colchicine (LD50 88.4 μΜ) [18][34].
5.2. Quinazolinone, Pyrazine, and Dioxopiperazine Alkaloids
Additionally, four new quinazolinone alkaloids, namely aniquinazolines A–D (74–77), were also purified from E. nidulans using SiO2/Sephadex LH-20/HPLC and elucidated by spectral analyses, and their absolute configurations were assigned based on acidic hydrolysate chiral HPLC, ECD, and X-ray analyses (Figure 7). Their configurations are 3R/14R/17R/18R/20S (74 and 76), 3R/14R/17R/18R (75), and 14R/17S/18S/20S (77). Compounds 74–77 had potent brine shrimp lethality (LD50s 1.27–4.95 μΜ) compared to colchicine (LD50 88.4 μΜ). Unfortunately, they did not have anticancer potential vs. MDA-MB-231, BEL-7402, K562, and HL-60 or antibacterial capacity vs. E. coli and S. aureus in the MTT and well diffusion assays, respectively [19][35].
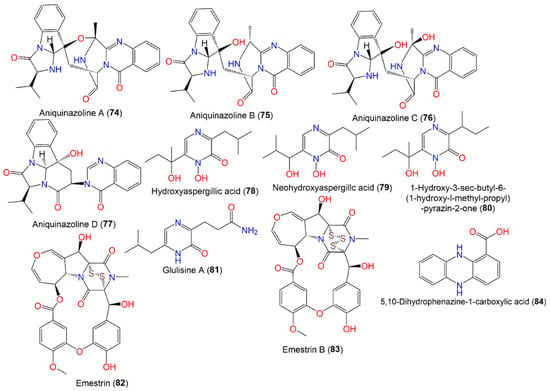
Figure 7.
Structures of quinazolinone (
74
–
77
), pyrazine (
78
–
81
), dioxopiperazine (
82
and
83
), and phenazine (
84
) alkaloids.
Emestrin (82), a six-membered-ring dioxopiperazine with a disulfide bridge, is part of the epipoly-thio-dioxopiperazine family obtained from ascidian Aplidium longithorax-associated E. nidulans MFW39 and collected from Wakatobi Marine National Park, Indonesia utilizing SiO2 CC/HPLC. It was found to have noticeable cytotoxic potential (IC50s ranged from 1.8 to 13.8 µg/mL) vs. C28, T47D, HeLa, and HepG2 but was weakly active against Vero cells (IC50 260.9 μg/mL). Emestrin (Conc. 1.0 μg/mL) caused G0/G1 arrest of the cell cycle, and also induced apoptosis (Conc. 1.0 and 3.0 μg/mL) of 83.6 and 92.6%, respectively, on T47D cells. This effect was linked to a disulphide bond and unique epithio-dioxopiperazine moiety [20][36]. Another epipolythiodioxopiperazine derivative, emestrin B (83), was separated from marine-derived E. nidulans using SiO2 CC/preparative TLC and was assessed for cytotoxic capacity in the MTT assay vs. the WiDr, HeLa, and T47D cancer cell lines. It had remarkable cytotoxic influence on IC50s 1.02, 1.56, and 0.16 μg/mL, respectively. This metabolite had apoptotic action against T47D cells (74.1%), compared to doxorubicin (74.8%) [21][37]. Compound 84, purified from the Nyctanthes arbor-tristis-inhabited fungus strain, had no cytotoxic effectiveness vs. the NCI-H460, MCF-7, and HeLa cell lines in the SRB [8][53]. Glulisine A (81), a new nitrogenous metabolite, was reported from 1% ethanol-stressed A. nidulans MA-143. It was assigned by NMR and X-ray analyses as being derived from leucine and glutamine [7][30].
5.3. Indole Derivatives
The indole alkaloid emindole DA (97), separated from green alga-associated fungus, possessed antitumor potential vs. 36 human tumor cell line panels (mean IC50s 5.5 µg/mL) in the propidium iodide fluorescence assay [1][43]. In an antimicrobial assay vs. various aquatic, plant, and human pathogens, terrequinone A (92) exhibited marked efficacy (MIC 2 μg/mL) vs. E. coli, V. alginolyticus, and C. gloeosporioides, compared to chloramphenicol and amphotericin B, suggesting the potency of 92 as a lead metabolite for antimicrobial agents [4][31]. Ergotryptamine (87) is an alkaloid produced by an engineered A. nidulans WFC that was designated using labeling, MS, and NMR tools. It differs from N-methyl-4-dimethylallyltryptophan (88) by the addition of the OH group, loss of the COOH group, and shifting of the double bond position [22][64] (Figure 8).
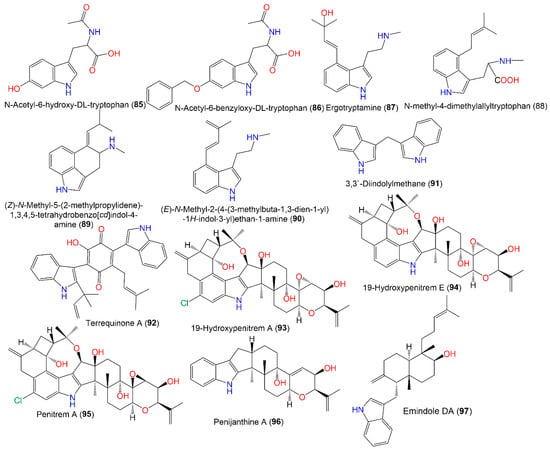
Figure 8.
Structures of indole (
85
–
97
) alkaloids.
5.4. Isoindole Derivatives
The production of new isoindole alkaloids, aspernidine A (99) and B (100 X2), by A. nidulans AXB4A2 cultivated in malt medium at increased orbital shaking (200 rpm) and elevated temperature (37 °C) with p-aminobenzoate/uridine supplementation, was reported. These metabolites were separated via SiO2/Sephadex LH-20/HPLC and characterized by MS and NMR tools (Figure 9). Compounds 99 and 100 had moderate anti-proliferation potential with respect to L-929 and K-562 (GI50 35.8/34.3 μM for 99 and 39.5/39.5 μM for 100, respectively) and weak cytotoxicity with respect to HeLa (CC50 94.0 and 65.5 μM, respectively) [23][65].
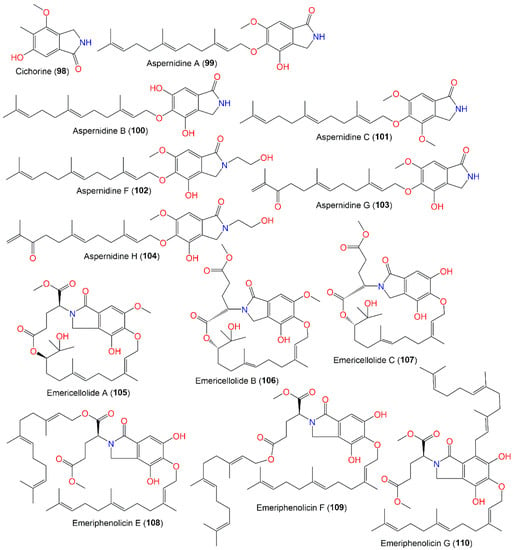
Figure 9.
Structures of isoindole (
98
–
110
) alkaloids.
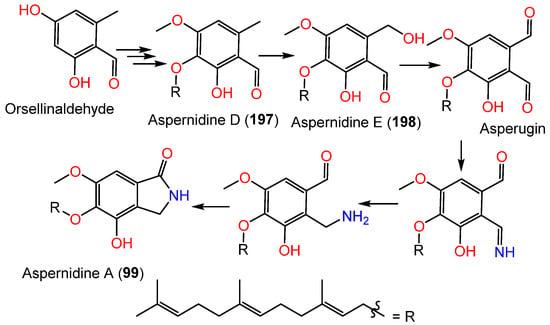
It was reported that biosynthesis of 99 starts with orsellinaldehyde from PKS-PkfA, which undergoes hydroxylation, one phenolic OH methylation, and prenylation to produce 197. Subsequently, hydroxylation of 197 yields 198 [24][49]. It was hypothesized that 198 is oxidized to produce asperugin, and then it is transformed into aspernidine A (99) through a series of steps [24][49] (Scheme 4).
From the red alga Polysiphonia scopulorum var. villum-associated A. nidulans EN-330, two new indole diterpenes are produced: 19-hydroxypenitrem A (93) and 19-hydroxypenitrem E (94), which are chlorinated and dechlorinated indole diterpenoids, respectively, along with known congeners 95 and 96, which were purified from the EtOAc cultures and acetone extracts by SiO2/Sephadex LH-20/HPLC and characterized by NMR and CD analyses. These metabolites feature an indole skeleton produced from a tryptophan precursor (indole-3-glycerol-phosphate) and geranyl-geranyl diphosphate-derived cyclic diterpenoid framework. Penitrem A (95) belongs to a rare tremorgenic mycotoxins group that has a C-19 skeleton fused to C-2 and C-3 of the indole moiety. Compound 93 is like 95, but it has C19-oxygenated quaternary carbon instead of the methine in 95. Both 93 and 94 are 19S/22S/31R/32S configured. In the brine shrimp assay, 93–96 possessed potent cytotoxic effectiveness (LD50s 3.2, 4.6, 1.7, and 8.7 μM, respectively), in comparison to colchicine (LD50 10.7 μM), where 93 and 95 were more powerful than 94, indicating that the C-6 Cl-substitution boosted the activity, while the 19-OH group suppressed it (93 vs. 95) [25][38]. In the assay for antibacterial activity against aquatic- (E. tarda and V. anguillarum) and human-pathogens (E. coli and S. aureus), 93–95 demonstrated moderate antimicrobial potential vs. E. tarda, V. anguillarum, E. coli, and S. aureus (MICs ranged from 16 to 64 μg/mL) [25][38].
From Annelida Whitmania pigra-associated A. nidulansi, aspernidines F–H (102–104), new prenylated isoindolinone alkaloids were purified and characterized utilizing SiO2/Sephadex LH-20/HPLC and spectral/ECD analyses, respectively. They possess a C5-linked C15 side chain. Compounds 103 and 104 exhibited moderate cytotoxic capacity on A-549, HL-60, SMMC-7721, SW-480, and MCF-7 (IC50s ranged from 4.77 to 33.03 μM) compared to cis-platin (IC50 3.22–22.96 μM). It was observed that 104 exhibited powerful inhibitory capacity vs. SW-480 cells (IC50s 4.77 μM) compared to cis-platin (IC50s 18.01 μM) [17][33].
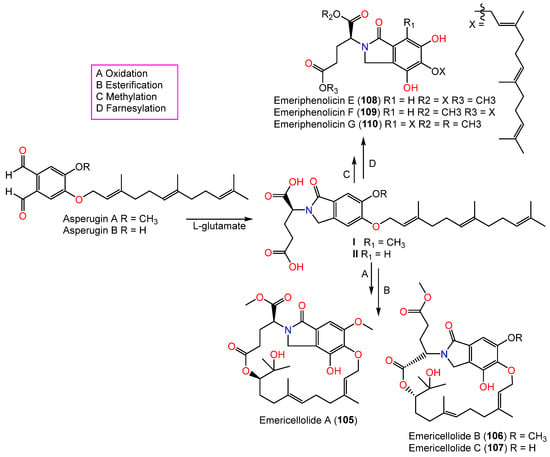
In 2016, novel meroterpenoids, emericellolides A–C (105–107) and emeriphenolicins E–G (108–110), with an isoindolone nucleus were separated from an A. nidulans HDN12.249 EtOAc broth extract isolated from Tamarix chinensis leaves that were obtained from Laizhou Bay using SiO2/RP-18/Sephadex LH-20/HPLC. Compounds 105–107 are characterized by an unparalleled macrolide skeleton consisting of an isoindolone moiety, an uncommon L-glutamate unit, and a sesquiterpene unit, while emeriphenolicins E–G (108–110) are rare isoindolone-based meroterpenoids with two farnesyl units linked to the isoindolone unit. These metabolites were structurally elucidated using NMR, MS, Mo2(AcO)4-induced ECD, chemical conversion, and Marfey’s method. In the SRB assay, only 108 exhibited selective potential vs. A549, HeLa, and HCT-116 (IC50s 12.04, 4.77, and 33.05 μM, respectively); however, the others were ineffective (IC50 > 50 μM) [26][39]. It was proposed that these metabolites were produced from asperugin A or B as an intermediate (Scheme 5). Further, farnesyl and L-glutamate units are formed through additional post-modifications, such as oxidation, methylation, and intra-molecular esterification [26][39].
5.5. Other Nitrogenous Compounds
Compound 111 was purified from cultured A. nidulans MeOH extract using alumina CC and crystallization from water. This compound had cytotoxic capacity vs. KB cells (endpoint value of 10–25 μ/mL and lethal endpoint of 50–100 μ/mL) [27][67], as well as inhibition of HCV NS3/4A protease (IC50 24.5 μg/mL), compared with HCV-12 (IC50 1.5 μg/mL), in addition to its potent cytotoxicity with inhibition zone difference of 100–200 mm vs. L1210, CCRF-CEM, colon 38, H-125, and HEP-G2 in the disc diffusion assay [6][47] (Figure 10).
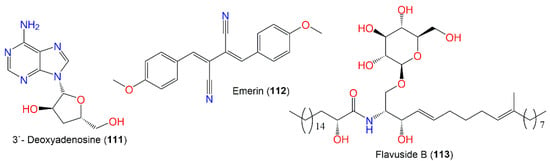
Figure 10.
Structures of other nitrogenous compound (
111
–
113
) alkaloids.
The cerebroside, flavuside B (113), reported from orange peel-associated E. nidulans, had no antimalarial, antimicrobial, or antileishmanial capacities [28][68].
6. Peptides
Diverse strategies have evolved to take advantage of the biosynthetic stockpile of fungi. Various strategies are reported to better utilize the BGC (biosynthetic genes cluster) of this fungus.
The identification of aspercryptins (116–133), a new family of lipopeptides via prohibition of histone deacetylase (HDACi) in A. nidulans, was reported. They were discovered by NMR and MS/MS metabolite screening (Figure 11, Figure 12, Figure 13 and Figure 14). These metabolites are six-amino-acid peptides, having two non-canonical α-amino acids derived from saturated C12 and C8 fatty acids and a C-terminal alcohol [29][69]. Hence, HDACi could be utilized for finding out new metabolites, even from fungal species that were intensively investigated.
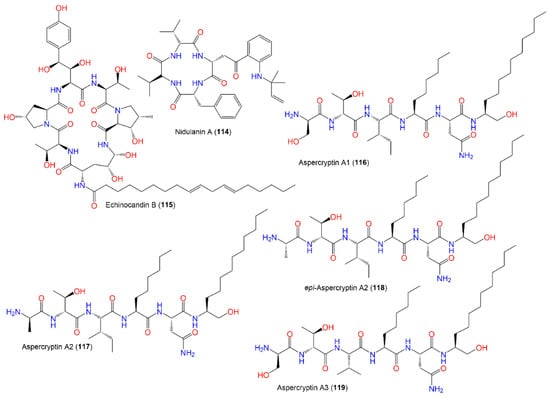
Figure 11.
Structures of peptides (
114
–
119
).
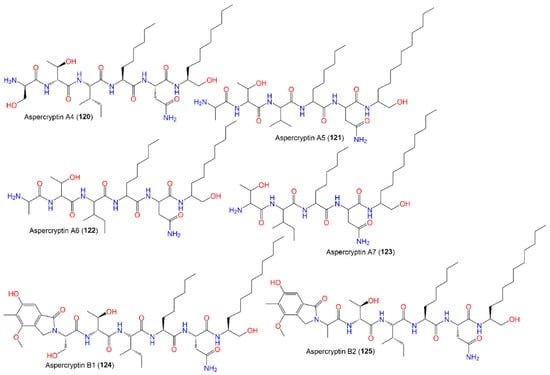
Figure 12.
Structures of peptides (
120
–
125
).
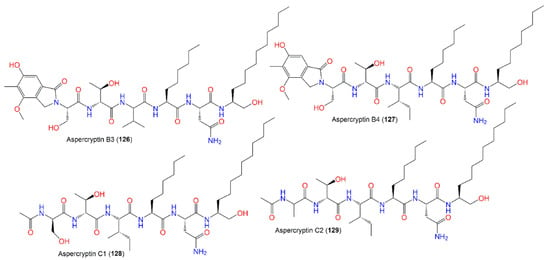
Figure 13.
Structures of peptides (
126
–
129
).
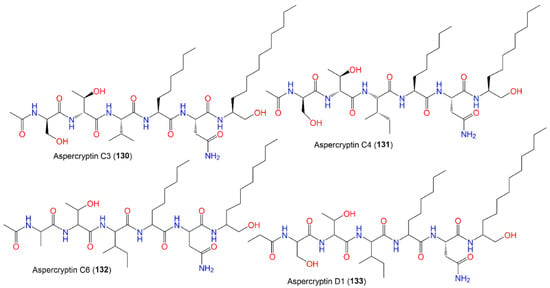
Figure 14.
Structures of peptides (
130
–
133
).
7. Terpenoids and Sterols
Terpenoids and sterols are among the metabolites reported from E. nidulans that are derived from mevalonate via a key step of cyclization and elimination reaction, respectively.
The engineered A. nidulans biosynthesized 134, which was extracted using ASE (accelerated solvent extraction) and purified by HPLC. In the DPPH assays, it had more powerful antioxidant potential than beta-carotene; however, it had no antimicrobial or anti-Leishmanial (50 µM) potential [30][71].
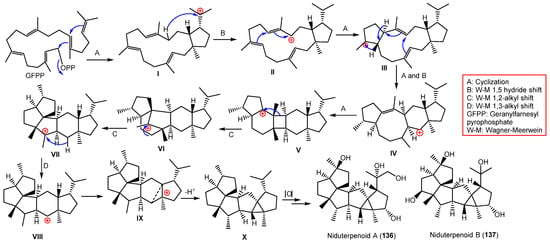
In 1983, β-amyrin was the first triterpenoid separated from A. nidulans [31][73]. From Whitmania pigra (Annelida, segmented worm)-associated A. nidulans, new sesterterpenoids, niduterpenoids A (136) and B (137), were reported that were purified from the EtOH culture extract by SiO2/Sephadex LH-20/HPLC (Figure 15). These metabolites were distinguished by a highly congested 5/5/5/5/3/5 hexacyclic skeleton without unsaturated functional groups, based on spectral and X-ray analyses [32][40]. They were assessed for their ERα (estrogen receptor α) inhibitory and cytotoxic potential vs. MCF-7 in the MTT assay. Compound 136 had no obvious cytotoxic potential, even at 80 μM, while it abolished the 17-estradiol-induced cell proliferation (IC50 11.42 μM) in a dose-based way. Molecular docking revealed that 136 and 137 fitted well with the ERα ligand-binding site and partially occupied the lasofoxifene (potent Er modulator) whole pocket. They formed key H-bonds with Arg346/Glu353/Met295 and a highly hydrophobic envelope with Leu298/Phe356/Phe377/Met294/Leu354. These findings indicated 136 was a potential ERα antagonist that required further studies [32][40].
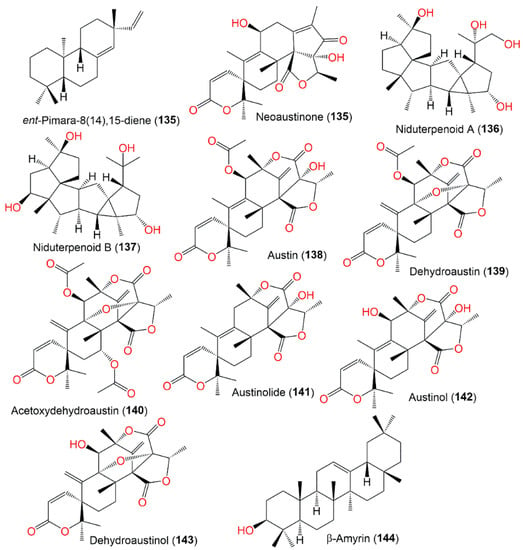
Figure 15.
Structures of terpenoids (
135
–
144
).
These metabolites are the first reported sesterterpenes with a hexacyclic ring system having an uncommon cyclopropane moiety (Scheme 6). It was proposed that they were biosynthesized from GFPP through various cyclization and Wagner–Meerwein alkyl shift and hydride reactions, producing the X (key intermediate) with a hexacyclic 5/5/5/5/3/5 framework. Additional oxidation reactions result in 136 and 137 [32][40].
Compounds 138, 139, and 142 are meroterpenoids separated from a cold spring, deep-sea-sediment-associated strain that had no antimicrobial capacity vs. aquatic/human bacteria and pathogenic plant fungi [4][31].
The separation of new metabolites 145 and 148–150 was reported, in addition to 146, 151–154, and 156 from the EtOAc extract of the fungus A. nidulans, utilizing SiO2/Sephadex LH-20/HPLC and elucidated by spectral, ECD, and X-ray analyses (Figure 16).
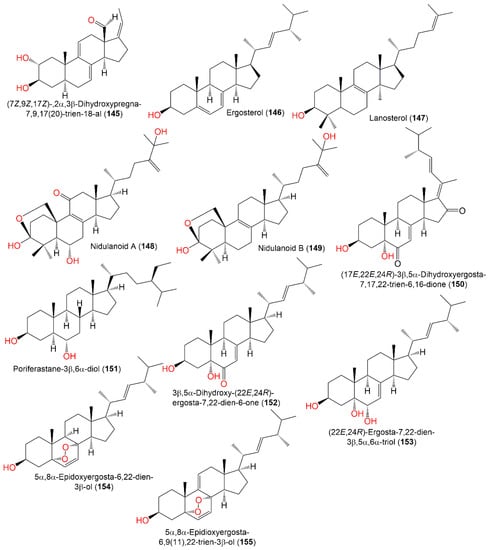
Figure 16.
Structures of sterols (
145
–
155
).
These metabolites were assessed for cytotoxic potential in vitro vs. the PC12 cell line in the MTT assay. Only 145 possessed moderate potential (IC50 7.34 µM) compared to doxorubicin (IC50 5.71 µM) [33][41]. Compound 167, reported from the EtOAc broth extract of Emericella nidulans, was mildly active as an HCV inhibitor (IC50 47.0 μg/mL) relative to HCV-12 (IC50 1.5 μg/mL) [6][47] (Figure 17).
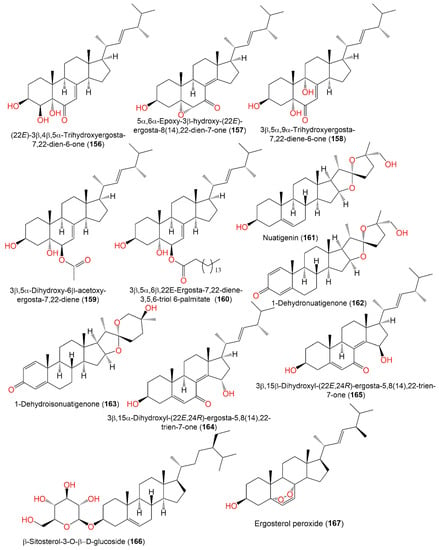
Figure 17.
Structures of sterols (
156
–
167
).
8. Lactones and Furanones
A new metabolite, 172, was separated from the acetone culture extract of A. nidulans IFO6398 by SiO2 CC using benzene as eluent. It featured two mono-substituted phenyl and maleic anhydride moieties; this compound was assigned by spectral analyses. In the radish seedlings bioassay, 172 accelerated root elongation (Conc. 30 and 100 g/L, respectively), while it had no notable influence on the hypocotyl elongation at the same concentrations. Moreover, 172 boosted root elongation and prohibited hypocotyl growth (Conc. 100 mg/L) in the lettuce seedling bioassay [34][75]. Compound 179, separated from the A. nidulans IFO6398 acetone extract, boosted root elongation (Conc. 30 g/L) and showed no notable effect on lettuce seedling growth (Conc. 3, 10, 30, and 100 mg/L) in the radish and lettuce seedling bioassays, respectively [34][75].
Microperfuranone (173), purified and characterized from E. nidulans var. acristata, had no antimicrobial, cytotoxic, and immune-stimulating activity [1][43], while 173 and 174 had antimicrobial potential against aquatic, human, and plant pathogens (MICs ranged from 4 to 64 μg/mL), whereas 173 presented notable effectiveness vs. E. ictarda (MIC 4 μg/mL) compared to chloramphenicol (MIC 2 μg/mL) [4][31] (Figure 18).
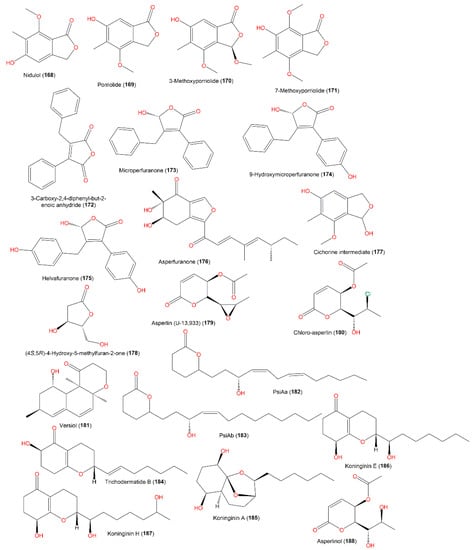
Figure 18.
Structures of furan (
168
–
178
) and pyran (
179
–
188
) derivatives.
9. Polyketides and Glycerides
A new polyketide, koninginin H (187), together with 184–186 and 193–196, was reported from E. nidulans isolated from an orange peel (Figure 19). Compound 187 belongs to the koninginin class of metabolites that was encountered in the Trichoderma genus [28][68]. An antimicrobial assessment of these metabolites vs. a panel of micro-organisms revealed that only 184 had good antifungal potential vs. C. neoformans (IC50 4.9 µg/mL) [28][68].
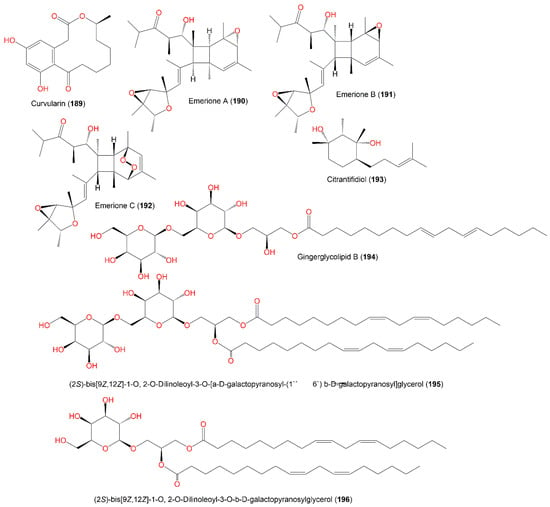
Figure 19.
Structures of polyketides (
189
–
193
) and glycerides (
194
–
196
).
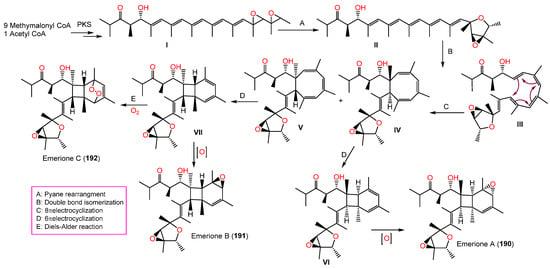
New metabolites, emeriones A-C (190–192), were purified from an EtOH extract of the culture broth of endophytic E. nidulans, obtained from Whitmania pigra by SiO2/Sephadex LH-20/HPLC. These metabolites are high-methylated polyketides with 3,6-dioxabicyclo[3.1.0]hexane and bicyclo[4.2.0]octene functionalities and were characterized using spectroscopic, X-ray, and CD analyses as well as ECD calculations. Compounds 190 and 191 are similar but differ in their configuration at the C9, C12, and C13 epoxy rings, possessing 2R/3R/4S/5S/8R/9S/12R/13S/14S/15S/16S/17R and 2R/3R/4S/5S/8S/9R/12S/13R/14R/15R/16S/17R configurations, respectively. Moreover, 192 has an extra peroxide bridge that results in an unusual 7,8-dioxatricyclo[4.2.2.02,5]decene scaffold construction. The NO production inhibitory efficacy of 190 and 191 was tested on LPS-mediated NO production in RAW264.7 using a CCK-8 assay. Interestingly, 190 demonstrated inhibition potential vs. NO production with a percent inhibition of NO production of 68.3% at a concentration of 10.0 μM, compared to indomethacin (with a percent inhibition of NO production of 84.2%, Conc. 40 μM); however, 191 had no effectiveness, although it possesses the same structure as 190 and differs only in the bicyclo[4.2.0]octane core absolute configuration [35][80]. They were assumed to be synthesized from methylmalonyl CoA (9 molecules) and acetyl-CoA (one molecule) by polyketide synthase to produce I (Scheme 7), which generates II via a Payne rearrangement that features a 3,6-dioxabicyclo[3.1.0]hexane moiety (Scheme 7).
An octatomic ring is then formed through 8π electrocyclization and double bond isomerization to produce IV and V, which undergo 6π electrocyclizations to yield VI and VII, with a pair of mirror-image bicyclo[4.2.0]-octene frameworks. Further, 190 and 191 are created through an additional C-12 double bond epoxidation, while 192 is formed through a Diels–Alder reaction between an O2 molecule and a bicyclo[4.2.0]octene core (Scheme 7) [35][80].
10. Other Metabolites
Asperbenzaldehyde (199) with an aromatic ring represents a new class of metabolites with tau aggregation inhibition potential. Moreover, its modified derivatives displayed noticeable lipoxygenase inhibition, suggesting its derived metabolites could exhibit dual activity [10][54].
The mycelia acetone extract of mutant A. nidulans LO2955 cultured on liquid lactose produced 202 (amount ≈200 mg/L), which was not found in such amounts in the wild strain culture. This compound had lipoxygenase-1 inhibition (IC50 97.2 μM) [37][42].
Asperoxide A (206), a new acyclic peroxide analog, and 203 were biosynthesized by A. nidulans SD531 obtained from cold spring, deep-sea sediment in the South China Sea by SiO2/RP-18 CC and characterized by NMR and MS analyses [4][31] (Figure 20). Their antimicrobial potential was examined vs. various aquatic/human bacteria and pathogenic plant fungi using a serial dilution technique. Compound 206 demonstrated antimicrobial effectiveness vs. V. harveyi, A. hydrophilia, V. parahaemolyticus, and E. tarda (MICs ranged from 16 to 64 μg/mL) [4][31].
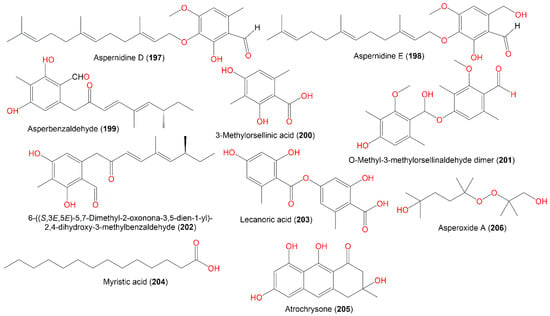
Figure 20.
Structures of other metabolites (
197
–
206
).