In the 1980s, non-A, non-B hepatitis (NANB) was identified to be hepatitis E virus (HEV), and found to be the causative agent of large scale viral hepatitis outbreaks in developing countries. One of the distinctive epidemiological features of an HEV outbreak is its association with a high pregnancy mortality rate, potentially reaching up to 35% mortality. HEV is ow recognized as the leading cause of acute viral hepatitis worldwide. HEV is a 7.2 kb quasi-enveloped, single-stranded RNA virus with a positive-sense genome. HEV has been difficult to grow in cell culture necessitating animal models to understand pathology. Multiple animal models have been attempted to replicate pregnancy mortality and those trials are summarized herein.
- hepatitis E
- pregnancy models
- rabbit
1. Background
2. HEV New Classification
3. Geographical Distribution of HEV
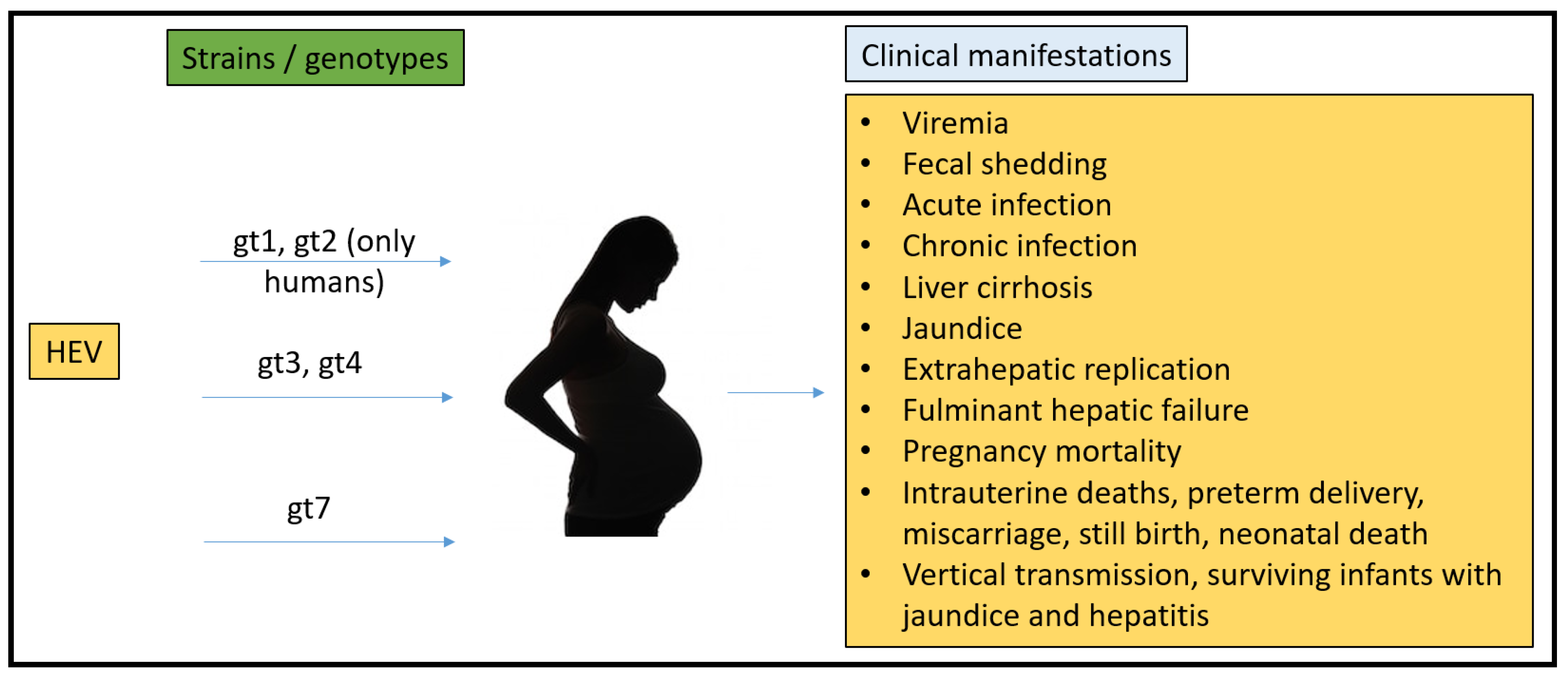
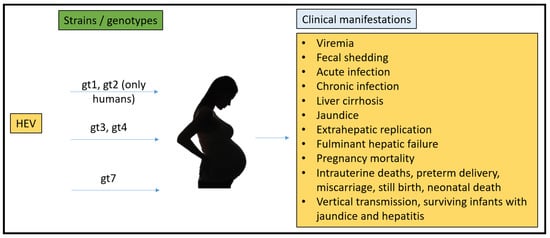
4. Pregnancy Mortality Reported Due to HEV
References
- Debing, Y.; Moradpour, D.; Neyts, J.; Gouttenoire, J. Update on hepatitis E virology: Implications for clinical practice. J. Hepatol. 2016, 65, 200–212.
- Feng, Z.; Hirai-Yuki, A.; McKnight, K.L.; Lemon, S.M. Naked viruses that aren’t always naked: Quasi-enveloped agents of acute hepatitis. Annu. Rev. Virol. 2014, 1, 539–560.
- Wong, D.C.; Purcell, R.H.; Sreenivasan, M.A.; Prasad, S.R.; Pavri, K.M. Epidemic and endemic hepatitis in India: Evidence for a non-A.; non-B hepatitis virus aetiology. Lancet 1980, 2, 876–879.
- Koonin, E.V.; Gorbalenya, A.E.; Purdy, M.A.; Rozanov, M.N.; Reyes, G.R.; Bradley, D.W. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: Delineation of an additional group of positive-strand RNA plant and animal viruses. Proc. Natl. Acad. Sci. USA 1992, 89, 8259–8263.
- Sridhar, S.; Teng, J.L.L.; Chiu, T.H.; Lau, S.K.P.; Woo, P.C.Y. Hepatitis E Virus Genotypes and Evolution: Emergence of Camel Hepatitis E Variants. Int. J. Mol. Sci. 2017, 18, 869.
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926.
- Xing, L.; Wang, J.C.; Li, T.C.; Yasutomi, Y.; Lara, J.; Khudyakov, Y.; Schofield, D.; Emerson, S.U.; Purcell, R.H.; Takeda, N.; et al. Spatial configuration of hepatitis E virus antigenic domain. J. Virol. 2011, 85, 1117–1124.
- Montpellier, C.; Wychowski, C.; Sayed, I.M.; Meunier, J.C.; Saliou, J.M.; Ankavay, M.; Bull, A.; Pillez, A.; Abravanel, F.; Helle, F.; et al. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology 2018, 154, 211–223.e218.
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778.
- Ding, Q.; Heller, B.; Capuccino, J.M.; Song, B.; Nimgaonkar, I.; Hrebikova, G.; Contreras, J.E. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc. Natl. Acad. Sci. USA 2017, 114, 1147–1152.
- Nair, V.P.; Anang, S.; Subramani, C.; Madhvi, A.; Bakshi, K.; Srivastava, A.; Shalimar Nayak, B.; Ranjith Kumar, C.T.; Surjit, M. Endoplasmic Reticulum Stress Induced Synthesis of a Novel Viral Factor Mediates Efficient Replication of Genotype-1 Hepatitis E Virus. PLoS Pathog. 2016, 12, e1005521.
- Tanggis Kobayashi, T.; Takahashi, M.; Jirintai, S.; Nishizawa, T.; Nagashima, S.; Nishiyama, T.; Kunita, S.; Hayama, E.; Tanaka, T.; Mulyanto Okamoto, H. An analysis of two open reading frames (ORF3 and ORF4) of rat hepatitis E virus genome using its infectious cDNA clones with mutations in ORF3 or ORF4. Virus Res. 2018, 249, 16–30.
- Yadav, K.K.; Boley, P.A.; Fritts, Z.; Kenney, S.P. Ectopic Expression of Genotype 1 Hepatitis E Virus ORF4 Increases Genotype 3 HEV Viral Replication in Cell Culture. Viruses 2021, 13, 75.
- Wang, B.; Yang, X.L. Chirohepevirus from Bats: Insights into Hepatitis E Virus Diversity and Evolution. Viruses 2022, 14, 905.
- Legrand-Abravanel, F.; Kamar, N.; Sandres-Saune, K.; Garrouste, C.; Dubois, M.; Mansuy, J.M.; Muscari, F.; Sallusto, F.; Rostaing, L.; Izopet, J. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J. Infect. Dis. 2010, 202, 835–844.
- Labrique, A.; Kuniholm, M.; Nelson, K. Emerging Infections; ASM Press: Washington, DC, USA, 2010.
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997.
- Teshale, E.H.; Hu, D.J.; Holmberg, S.D. The two faces of hepatitis E virus. Clin. Infect. Dis. 2010, 51, 328–334.
- Meng, X.J. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011, 161, 23–30.
- Teo, C.G. Much meat, much malady: Changing perceptions of the epidemiology of hepatitis E. Clin. Microbiol. Infect. 2010, 16, 24–32.
- Kuniholm, M.H.; Purcell, R.H.; McQuillan, G.M.; Engle, R.E.; Wasley, A.; Nelson, K.E. Epidemiology of hepatitis E virus in the United States: Results from the Third National Health and Nutrition Examination Survey, 1988–1994. J. Infect. Dis. 2009, 200, 48–56.
- Nelson, K.E.; Kmush, B.; Labrique, A.B. The epidemiology of hepatitis E virus infections in developed countries and among immunocompromised patients. Expert Rev. Anti. Infect. Ther. 2011, 9, 1133–1148.
- Xia, H.; Wahlberg, N.; Belak, S.; Meng, X.J.; Liu, L. The emergence of genotypes 3 and 4 hepatitis E virus in swine and humans: A phylogenetic perspective. Arch. Virol. 2011, 156, 121–124.
- Chandra, V.; Taneja, S.; Kalia, M.; Jameel, S. Molecular biology and pathogenesis of hepatitis E virus. J. Biosci. 2008, 33, 451–464.
- Pavio, N.; Meng, X.-J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46.
- Chauhan, A.; Webb, G.; Ferguson, J. Clinical presentations of Hepatitis E: A clinical review with representative case histories. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 649–657.
- Rab, M.A.; Bile, M.K.; Mubarik, M.M.; Asghar, H.; Sami, Z.; Siddiqi, S.; Dil, A.S.; Barzgar, M.A.; Chaudhry, M.A.; Burney, M.I. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: A common source outbreak traced to the malfunction of a modern water treatment plant. Am. J. Trop. Med. Hyg. 1997, 57, 151–157.
- Teo, C.G. Fatal outbreaks of jaundice in pregnancy and the epidemic history of hepatitis E. Epidemiol. Infect. 2012, 140, 767–787.
- Greenberg, M. Hepatitis Frontiers; American Public Health Association: Washington, DC, USA, 1958.
- Boccia, D.; Guthmann, J.P.; Klovstad, H.; Hamid, N.; Tatay, M.; Ciglenecki, I.; Nizou, J.Y.; Nicand, E.; Guerin, P.J. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin. Infect. Dis. 2006, 42, 1679–1684.
- Arankalle, V.A.; Chobe, L.P. Hepatitis E virus: Can it be transmitted parenterally? J. Viral Hepat. 1999, 6, 161–164.
- Khuroo, M.S.; Kamali, S.; Jameel, S. Vertical transmission of hepatitis E virus. Lancet 1995, 345, 1025–1026.
- Kumar, R.M.; Uduman, S.; Rana, S.; Kochiyil, J.K.; Usmani, A.; Thomas, L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 100, 9–15.
- Khuroo, M.S.; Kamili, S.; Khuroo, M.S. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J. Viral Hepat. 2009, 16, 519–523.
- Purcell, R.H. The Hepatitis Viruses: An Overview. In Viral Hepatitis and Liver Disease; Nishioka, K., Suzuki, H., Mishiro, S., Oda, T., Eds.; Springer: Tokyo, Japan, 1994; pp. 19–21.
- Arankalle, V.A.; Chadha, M.S.; Tsarev, S.A.; Emerson, S.U.; Risbud, A.R.; Banerjee, K.; Purcell, R.H. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA 1994, 91, 3428–3432.
- Purdy, M.A.; Khudyakov, Y.E. Evolutionary history and population dynamics of hepatitis E virus. PLoS ONE 2010, 5, e14376.
- Purdy, M.A.; Khudyakov, Y.E. The molecular epidemiology of hepatitis E virus infection. Virus Res. 2011, 161, 31–39.
- Naidu, S.S.; Viswanathan, R. Infectious hepatitis in pregnancy during Delhi epidemic. Indian J. Med. Res. 1957, 45, 71–76.
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a virus in non-A.; non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983, 20, 23–31.
- Patra, S.; Kumar, A.; Trivedi, S.S.; Puri, M.; Sarin, S.K. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann. Intern. Med. 2007, 147, 28–33.
- Beniwal, M.; Kumar, A.; Kar, P.; Jilani, N.; Sharma, J.B. Prevalence and severity of acute viral hepatitis and fulminant hepatitis during pregnancy: A prospective study from north India. Indian J. Med. Microbiol. 2003, 21, 184–185.
- Tsega, E.; Hansson, B.G.; Krawczynski, K.; Nordenfelt, E. Acute sporadic viral hepatitis in Ethiopia: Causes, risk factors, and effects on pregnancy. Clin. Infect. Dis. 1992, 14, 961–965.
- Goumba, C.M.; Yandoko-Nakoune, E.R.; Komas, N.P. A fatal case of acute hepatitis E among pregnant women, Central African Republic. BMC Res. Notes 2010, 3, 103.
- ICDDRB. Outbreak of hepatitis E in a low income urban community in Bangladesh. Health Sci. Bull. 2009, 7, 14–20.
- Rasheeda, C.A.; Navaneethan, U.; Jayanthi, V. Liver disease in pregnancy and its influence on maternal and fetal mortality: A prospective study from Chennai, Southern India. Eur. J. Gastroenterol. Hepatol. 2008, 20, 362–364.
- Rayis, D.A.; Jumaa, A.M.; Gasim, G.I.; Karsany, M.S.; Adam, I. An outbreak of hepatitis E and high maternal mortality at Port Sudan, Eastern Sudan. Pathog. Glob. Health 2013, 107, 66–68.
