The potential of insects as a sustainable and easily accessible source of chitin and chitosan is highlighted in “Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector”. Researchers describe the state of the art in the extraction and purification of chitin and chitosan from edible insects. Chitin and chitosan may be extracted and purified from edible insect species like the black soldier fly and the cricket since they are naturally rich in chitin. Due to their strong biological activity, chitin and chitosan have many potential uses in the agriculture, food, and biomedical industry sectors. Insect chitin and chitosan might be utilised to provide a sustainable and environmentally friendly alternative to petroleum-based polymers in food packaging. Chitin and chitosan have been shown to have antibacterial characteristics, making them valuable for a variety of biological applications. Insects' tremendous potential as a renewable and profitable resource for chitin and chitosan. Let's continue to explore and develop these innovative solutions for a more sustainable future!
- antibacterial
- antioxidants
- food packaging
- food shelf lives
- black soldier fly
1. Chitin and Chitosan Sources
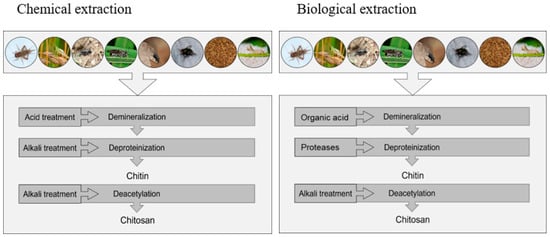
2. Methods for Chitin and Chitosan Extraction
| # | Method | Methods of Treatment | Advantages and Disadvantages |
|---|---|---|---|
| 1 | Chemical extraction | Demineralization: by acidic treatment using HCl, HNO3, H2SO4.Deproteinization: by alkaline treatment using NaOH or KOH.Decoloration: acetone or organic solvent.Deacetylation: by alkaline treatment using a strong NaOH or KOH |
|
| 2 | Biological extraction | Demineralization: Bacteria-produced lactic acid is used in demineralization.Deproteinization: Fermentation media proteases are released into the medium to deproteinize the cultureDecoloration: Acetone or organic solvents are effective decolorizers.Deacetylation: Bacteria produce chitin deacetylase, which deacetifies chitin. |
|
References
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25.
- Tacon, A.G.J. Global Trends in Aquaculture and Compound Aquafeed Production; World Aquaculture Society: Stavanger, Norway, 2018.
- Terkula Iber, B.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew Mater. 2022, 10, 1097–1123.
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036.
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of Chitin and Chitosan and Their Isolation. In Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 1–34.
- Rana, K.L.; Kour, D.; Sheikh, I.; Dhiman, A.; Yadav, N.; Yadav, A.N.; Rastegari, A.A.; Singh, K.; Saxena, A.K. Endophytic Fungi: Biodiversity, Ecological Significance, and Potential Industrial Applications. In Recent Advancement in White Biotechnology through Fungi, Volume 1: Diversity and Enzymes Perspectives; Springer: Berlin, Germany, 2019.
- Fernando, L.D.; Dickwella Widanage, M.C.; Penfield, J.; Lipton, A.S.; Washton, N.; Latgé, J.P.; Wang, P.; Zhang, L.; Wang, T. Structural Polymorphism of Chitin and Chitosan in Fungal Cell Walls from Solid-State NMR and Principal Component Analysis. Front. Mol. Biosci. 2021, 8, 814.
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Diversity, Taxonomy and Phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137.
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan:: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98.
- Hazmi, A.T.; Ahmad, F.B.; Maziati Akmal, M.H.; Md Ralib, A.A.; Binti Ali, F. Fungal Chitosan for Potential Application in Piezoelectric Energy Harvesting: Review on Experimental Procedure of Chitosan Extraction. Alex. Eng. J. 2022, 67, 105–116.
- Crognale, S.; Russo, C.; Petruccioli, M.; D’annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76.
- Shahbaz, U. Chitin, Characteristic, Sources, and Biomedical Application. Curr. Pharm. Biotechnol. 2020, 21, 1433–1443.
- el Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and Efficient Extraction of Chitin and Chitosan for Scale-up Production: Effect of Process Parameters on Deacetylation Degree and Molecular Weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102.
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614.
- Mathew, G.M.; Sukumaran, R.K.; Sindhu, R.; Binod, P.; Pandey, A. Green Remediation of the Potential Hazardous Shellfish Wastes Generated from the Processing Industries and Their Bioprospecting. Environ. Technol. Innov 2021, 24, 101979.
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin Extraction from Shrimp Waste by Liquid Fermentation Using an Alkaline Protease-Producing Strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715.
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50.
- Gonil, P.; Sajomsang, W. Applications of Magnetic Resonance Spectroscopy to Chitin from Insect Cuticles. Int. J. Biol. Macromol. 2012, 51, 514–522.
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846.
- Bradić, B.; Novak, U.; Likozar, B. Crustacean Shell Bio-Refining to Chitin by Natural Deep Eutectic Solvents. Green Process Synth. 2020, 9, 13–25.
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; de Franco, L.O.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290.
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of Chitosan and Its Oligomers from Shrimp Shell Waste, Their Characterization and Antimicrobial Effect. Vet. World 2017, 10, 170–175.
- Roberts, G.A.F. Preparation of Chitin and Chitosan. In Chitin Chemistry; Palgrave: London, UK, 1992; pp. 54–84.
- Kostag, M.; el Seoud, O.A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079.
- Kou, S. Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94.
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin, Chitosan and Their Melanin Complexes from Insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328.
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Van den Broek, L.A.M.; Boeriu, C.G. Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2019; ISBN 9781119450467.
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709.
- Hussain, R.; Maji, T.K.; Maji, T.K. Determination of Degree of Deacetylation of Chitosan and Their Effect on the Release Behavior of Essential Oil from Chitosan and Chitosan-Gelatin Complex Microcapsules. Int. J. Adv. Eng. Appl. 2013, 2, 4–12.
- Mukarram, M.; Naeem, M.; Aftab, T.; Khan, M.M.A. Chitin, Chitosan, and Chitooligosaccharides: Recent Advances and Future Perspectives. In Radiation-Processed Polysaccharides; Academic Press: Cambridge, MA, USA, 2022; pp. 339–353.
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337.
- Anand, M.; Kalaivani, R.; Maruthupandy, M.; Kumaraguru, A.K.; Suresh, S. Extraction and Characterization of Chitosan from Marine Crab and Squilla Collected from the Gulf of Mannar Region, South India. J. Chitin Chitosan Sci. 2014, 2, 280–287.
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green Synthesis of Chitosan-Cinnamaldehyde Cross-Linked Nanoparticles: Characterization and Antibacterial Activity. Carbohydr. Polym. 2019, 226, 115298.
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers 2021, 13, 2256.
- Shirai, K.; Guerrero, I.; Huerta, S.; Saucedo, G.; Castillo, A.; Obdulia Gonzalez, R.; Hall, G.M. Effect of Initial Glucose Concentration and Inoculation Level of Lactic Acid Bacteria in Shrimp Waste Ensilation. Enzym. Microb. Technol. 2001, 28, 446–452.
- De Holanda, H.D.; Netto, F.M. Recovery of Components from Shrimp (Xiphopenaeus kroyeri) Processing Waste by Enzymatic Hydrolysis. J. Food Sci. 2006, 71, C298–C303.
- Van Nguyen, N.; Hai, P.D.; My My, V.T.; Men, D.T.; Trung, L.D.; Bavor, H.J. Improving Product Added-Value from Shrimp (Litopenaeus vannamei) Waste by Using Enzymatic Hydrolysis and Response Surface Methodology. J. Aquat. Food Prod. Technol. 2021, 30, 880–892.
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17.
- Rao, M.S.; Muñoz, J.; Stevens, W.F. Critical Factors in Chitin Production by Fermentation of Shrimp Biowaste. Appl. Microbiol. Biotechnol. 2000, 54, 808–813.
- Yang, J.K.; Shih, I.L.; Tzeng, Y.M.; Wang, S.L. Production and Purification of Protease from a Bacillus Subtilis That Can Deproteinize Crustacean Wastes. Enzym. Microb. Technol. 2000, 26, 406–413.
- Gartner, C.; Peláez, C.A.; López, B.L. Characterization of Chitin and Chitosan Extracted from Shrimp Shells by Two Methods. E-Polymers 2010, 10, 69.
- Trung, T.S.; Tram, L.H.; van Tan, N.; van Hoa, N.; Minh, N.C.; Loc, P.T.; Stevens, W.F. Improved Method for Production of Chitin and Chitosan from Shrimp Shells. Carbohydr. Res 2020, 489, 107913.
- Hongkulsup, C.; Khutoryanskiy, V.-v.; Niranjan, K. Enzyme Assisted Extraction of Chitin from Shrimp Shells (Litopenaeus Vannamei). J. Chem. Technol. Biotechnol. 2016, 91, 1250–1256.
- Valdez-Peña, A.U.; Espinoza-Perez, J.D.; Sandoval-Fabian, G.C.; Balagurusamy, N.; Hernandez-Rivera, A.; De-la-Garza-Rodriguez, I.M.; Contreras-Esquivel, J.C. Screening of Industrial Enzymes for Deproteinization of Shrimp Head for Chitin Recovery. Food Sci. Biotechnol. 2010, 19, 553–557.
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable Ecofriendly Phytoextract Mediated One Pot Green Recovery of Chitosan. Sci. Rep. 2019, 9, 1–12.
- Singh, S.K. Solubility of Lignin and Chitin in Ionic Liquids and Their Biomedical Applications. Int. J. Biol. Macromol. 2019, 132, 265–277.
- Morais, E.S.; da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652.
- Idenoue, S.; Yamamoto, K.; Kadokawa, J.I. Dissolution of Chitin in Deep Eutectic Solvents Composed of Imidazolium Ionic Liquids and Thiourea. Chem. Eng. 2019, 3, 90.
- Ramón, D.J.; Guillena, G. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley: Hoboken, NJ, USA, 2019; ISBN 9783527818471.
- Huang, W.C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and Facile Production of Chitin from Crustacean Shells Using a Natural Deep Eutectic Solvent. J. Agric. Food Chem. 2018, 66, 11897–11901.
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile Acid Base Sustainable Solvent for Fast Extraction of Various Molecular Weight Chitin from Lobster Shell. Carbohydr. Polym. 2018, 201, 211–217.
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and Physicochemical Properties of Chitin Polymer from Insect Farm Side Stream as a New Source of Renewable Biopolymer. J. Clean. Prod. 2020, 275, 122924.
