13C solid-state Nuclear Magnetic Resonance (SSNMR) has often been applied to follow the transformation of organic matter during waste composting to produce soil amendments, as well as to assess the quality of the products and the effectiveness of the treatment. Here we present a review of the relevant literature to this topic, highlighting the potential of the 13C SSNMR technique, as well as the critical issues and perspectives.
- humic substances
- humic acids
- 13C CP-MAS
1. Introduction
2. 13C Solid-State NMR Spectroscopy for the Investigation of Organic Matter in Compost
13C has a natural abundance of 1.11% [25],[26], which results in a good balance of sensitivity and spectral “simplicity” since complex interactions among 13C nuclei can be neglected. The most important nuclear property used for the identification of functional groups is chemical shielding. On the basis of this property, nuclei in different chemical environments give rise to signals at different resonance frequencies, expressed as chemical shifts. Experimental techniques providing high-resolution 13C SSNMR spectra are at present routinely available on SSNMR instruments, the most important being Magic Angle Spinning (MAS) and high-power decoupling from abundant 1H nuclei. In the most basic experiment, usually called direct polarization MAS or direct excitation MAS (DE-MAS), these techniques are combined with a radiofrequency excitation pulse on 13C. The 13C DE-MAS experiment gives the most accurate quantification of the different species in an OM solid sample, provided that it is performed with a sufficiently long recycle delay between consecutive scans (five times the spin-lattice relaxation time, T1, of the 13C nuclei). Since T1 values for 13C nuclei in the solid state can be considerably long (even hundreds of seconds), extremely long measurement times are required for acquiring quantitative 13C DE-MAS spectra with an acceptable signal-to-noise ratio. For this reason 1H-13C cross polarization MAS (CP-MAS) experiments are most commonly used in the investigation of OM samples. In CP-MAS experiments, the 13C signal is created by magnetization transfer from the abundant 1H nuclei during a time interval called contact time, with an enhancement by up to a factor of four. Moreover, in CP experiments the recycle delay is determined by the longitudinal relaxation of the 1H nuclei, which is usually much faster than that of 13C. This allows recycle delays of a few seconds to be used, with a substantial reduction in the experimental time. It is worth noting that, while CP-MAS is an excellent technique for investigating the qualitative composition of a sample, the relative peak intensities may not be fully preserved. However, it has been reported that in the case of compost 13C CP-MAS NMR spectra recorded with a contact time of about 1 ms and a recycle delay of a few seconds show a similar intensity loss (<10%), with respect to the quantitative spectrum, for signals of all functional groups [27], allowing these conditions to be used for relative quantitation. This also generally applies in the comparison of a series of spectra of samples with similar composition. Therefore, 13C CP-MAS NMR experiments with a contact time of 1 ms and a recycle delay of a few seconds are usually employed in works on OM in composts to investigate and compare OM composition in the feedstock (biomasses or wastes) and throughout (or only at the end of) the decomposition process.|
Chemical shift range |
Assignment |
Conventional region name |
||
|
0-28 ppm |
CH3 and CH2 in short chain and simple aliphatics |
Alkyl C |
||
|
28-45 ppm |
CH2 and CH in long aliphatic chains |
|||
|
45-60 ppm |
O-CH3; CH-N; aliphatic quaternary C |
O-Alkyl C |
||
|
60-95 ppma |
C2-C6 in cellulose and hemicellulose; alcohols; ethers |
|||
|
95-110 ppmb |
C1 of cellulose and hemicellulose; anomeric carbon of polysaccharides |
|||
|
110-145 ppmc |
Unsubstituted or alkyl-substituted aromatic C |
Aromatic C |
||
|
145-160 ppmd |
O,N-substituted aromatics |
|||
|
160-190 ppm |
Carboxylic acids; esters; amides |
Carboxyl/carbonyl C |
||
|
190-220 ppm |
Ketones; aldehydes |
|||
aalso referred to as O-alkyl C region; balso referred to as di-O-alkyl C region; calso referred to as aryl C region; dalso referred to as O-aryl C region.
Although 13C CP-MAS NMR is semiquantitative, the integral values of different spectral regions (expressed as % of total area) have been extensively used as quantitative proxies for the relative C distribution among major OM functional groups in the feedstocks and in the intermediate/final products, thus monitoring the transformation process of the different components. It must be pointed out that, although most authors do not give errors on the integral intensities and some even report integral values with one or two decimal digits, errors on the units digit are to be expected, deriving from sampling and spectral processing. Moreover, normally, relative integral intensities are compared without taking into account the decrease in the absolute amount of carbon in OM due to decomposition.
Different indices are also used to express OM composition, the most common ones being the Alkyl C/O-Alkyl C (A/OA) ratio, the aromaticity index (ARM), and the hydrophobicity index (HB/HI), generally defined as:
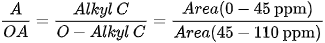
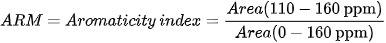
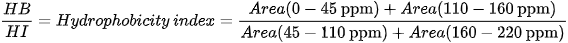
13C SSNMR experiments on OM samples in waste management are generally carried out on spectrometers working at 13C Larmor frequencies of 75-125 MHz, although a frequency of 150 MHz is used in more recent studies and frequencies as low as 25 MHz were employed in the older ones. MAS rotors with diameters of 4 mm, containing few tens of milligrams of sample, and MAS frequencies of 8-13 kHz are usually employed. Few cases are also reported where rotors with 7 mm diameter and spinning rates of 3.5-5 kHz were used. As previously said, 13C CP-MAS experiments are usually performed with a contact time of 1 ms, although cases are reported where longer contact times (2-3 ms) are used. In these experiments, the recycle delay ranges from 0.5 to 5 s and few thousands of scans are acquired.
Before 13C SSNMR experiments, OM samples are simply dried (by freeze-drying, oven-drying at 40-110°C, or air-drying), ground, and possibly sieved to obtain a fine powder; the latter operation is not strictly necessary, but it is useful since it ensures stable sample spinning and, consequently, a better spectral resolution. Considering that only few tens of milligrams of powder are necessary for the analysis, accurate sampling and homogenization are critical to obtain a representative sample.
Several studies on composts, however, instead of investigating the bulk material, focused on extracts. Different types of extracts were investigated, that is, humic substances (HSs), dissolved organic matter (DOM), or compost tea (CT). Humic substances are stable compounds with a complex and variable composition, mainly containing aromatic rings linked by methylenic chains and/or oxygen atoms, with carboxyl and hydroxyl groups bonded to the rings and the alkyl chains. On the basis of their solubility, two different extractable HS fractions can be obtained, i.e. fulvic acids (FAs, soluble at any pH value) and humic acids (HAs, soluble at pH>2). The extraction of HSs is performed mixing the dry compost in an alkaline solution; HAs can then be separated by acidifying to pH 1.0. HAs have higher molecular weight and degree of aromatization with respect to FAs, which, on the other hand, are richer in carboxyl and hydroxyl groups [35]. Thanks to their highly aromatic structure, HAs are stable compounds and their amount is typically considered as a measure of compost maturity [36]. In fact, during composting HAs tend to increase whereas FAs tend to decrease [37]. Compost DOM is a mixture of low-molecular weight compounds, such as sugars and free amino acids, and high-molecular weight compounds, among which also HSs [38]. DOM is a very small fraction of the total organic matter present in compost, decreasing as feedstock stabilization proceeds, but it is an important component since many biochemical transformations that occur during composting take place in solution. DOM is extracted from compost by shaking the material with ultrapure water; DOM can be further divided in a hydrophobic fraction (HoDOM) and a hydrophylic fraction (HiDOM) using the Amberlite® XAD-8 or Supelite™ DAX-8 resin [38]. Finally, compost tea is an aqueous extract obtained from the fermentation of compost in water either in forced aerated or non-aerated conditions [39].
3. 13C Solid-State NMR Applications to Composting
The literature reports many examples of the application of 13C MAS NMR to the investigation of compost, covering a very broad range of diverse feedstocks, including, often mixed, animal (cattle, swine, chicken, buffalo) manure [40][41][42][43][44][45][46][47][48][49][50][51][52], vegetal residues [10][41][42][52][53][54][55][56][57][58], grape marc [50][59], ground coffee and fresh grass [10][57], cotton gin waste [45], rice husk, bran or straw and wheat straw [44][46][56], domestic organic wastes [55][57][60], and municipal solid wastes (MSW) [27][61][62]. The composting process has been followed by means of 13C CP-MAS NMR for different times, ranging from a few days, for specifically investigating the initial part of the process [10], up to more than one year [46]. An example of the evolution of the 13C CP-MAS spectrum of different feedstocks with composting time is shown in Figure 1. In most cases the reported experimental data are the integral areas of the characteristic spectral regions described in Section 2. These data are catalogued in Table 2, indicating for each study the feedstock and the composting time.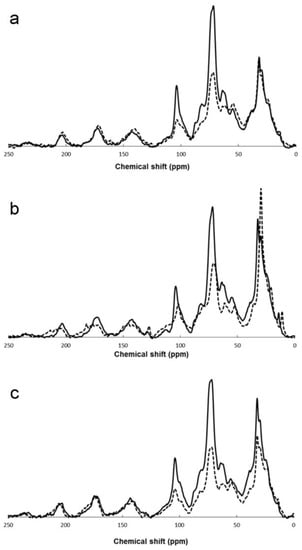
| Ref. a | Feedstock b | Sample c | Alkyl C | O-Alkyl C | Aromatic C | Carboxyl/ Carbonyl C |
||
|---|---|---|---|---|---|---|---|---|
| Ref. a | Feedstock b | Sample c | Alkyl C | O-Alkyl C | Aromatic C | Carboxyl/ Carbonyl C |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH | 3O/CHN | O-/Di-O-alkyl C | Aryl C | O-Aryl C | |||||||||||||
| CH3O/CHN | O-/Di-O-Alkyl C | Aryl C | O-Aryl C | Carboxyl C | Carbonyl C | ||||||||||||
| [41] | Olive mill waste/orchard pruning residues | C 200 d | 17.39 | 11.63 | 45.86 | 12.31 | 5.75 | 7.06 | |||||||||
| [72] | SM/poplar sawdust (5:3 w:w) | HA 60 d | 30.3 | 33.9 | 16.8 | 7.1 | 11.9 | ||||||||||
| Olive mill waste/animal manure/wool residues | C 200 d | 26.82 | 15.32 | 38.59 | 6.93 | 4.0 | 8.34 | ||||||||||
| [52] | ChM/saw dust (3:1 w:w) | C 56 d | 28.38 | 32.26 | 16.51 | ||||||||||||
| CM/saw dust (3:1 w:w | |||||||||||||||||
| +sepiolite 3 wt% | HA 60 d | 26.7 | 29.9 | 18.3 | 8.4 | 16.8 | ) | ||||||||||
| +sepiolite 6 wt% | HA 60 d | 34.7 | 22.6 | 17.4 | 7.9 | 17.4 | C 56 d | 22.13 | 32.20 | 10.49 | |||||||
| +sepiolite 9 wt% | HA 60 d | 24.3 | 29.4 | 18.3 | 10.4 | 17.6 | SM/saw dust (3:1 w:w) | ||||||||||
| +sepiolite 12 wt% | C 56 d | HA 60 d31.62 | 24.0 | 41.36 | 29.917.71 | ||||||||||||
| 20.8 | 8.7 | 16.5 | Soybean meal/saw dust (3:1 w:w) | C 56 d | 37.94 | 43.16 | 18.40 | ||||||||||
| [69] | CYN/corn straw and WC (70:30 w:w) | HS 100 d | 20.93 | 15.98 | 29.78 | 16.26 | 5.51 | 11.54 | Lemon peel/saw dust (3:1 w:w) | C 56 d | 36.23 | 41.10 | 17.43 | ||||
| COF/corn straw and WC (70:30 w:w) | HS 100 d | 30.63 | 13.07 | 24.69 | 15.33 | 3.42 | 12.85 | [58] | |||||||||
| PEP/corn straw and WC (70:30 w: | Wood chips/vegetable R/aromatic plant R | wC1 | 33.9 | 13.0 | 31.3 | ) | HS 100 d | 20.06 | 14.2212.6 | 2.1 | 7.2 | ||||||
| 35.75 | 14.40 | 4.49 | 11.08 | C2 | 38.2 | 12.3 | 26.8 | 11.5 | 2.9 | 8.2 | |||||||
| [87] | CYN/WC (70:30 w:w) | HA 100 d | 22.2 | 13.8 | 27.5 | 20.0 | 5.7 | 10.9 | C3 | 23.4 | 11.7 | 44.0 | |||||
| [ | 12.1 | 3.7 | 5.1 | ||||||||||||||
| 43] | SM/rice straw 4:1 | HA FS | 46.8 | 19.6 | 18.1 | 15.5 | C4 | 21.0 | 11.8 | 41.1 | |||||||
| 14.3 | 3.8 | 8.1 | |||||||||||||||
| HA 40d | 28.4 | 21.7 | 31.3 | 18.7 | C5 | 19.0 | 11.4 | 42.4 | 15.5 | 3.8 | 7.9 | ||||||
| 22.2 | |||||||||||||||||
| SM/rice straw 8:1 | HA FS | 45.8 | 17.2 | 14.7 | 22.3 | C6 | 24.6 | 10.9 | 38.7 | 15.1 | |||||||
| HA 40d | 28.9 | 3.3 | 26.8 | 7.4 | |||||||||||||
| 31.6 | C7 | 35.7 | 11.8 | 31.0 | 11.8 | 2.5 | 7.2 | ||||||||||
| 12.7 | |||||||||||||||||
| SM/rice straw/biochar 8:1:1 | HA FS | 43.2 | 19.9 | 18.4 | 18.5 | C8 | 37.8 | 12.0 | 30.1 | 11.4 | 2.2 | 6.5 | |||||
| HA 40d | 27.9 | 23.4 | 30.5 | 18.3 | C9 | 43.6 | 10.5 | 28.0 | 9.5 | 2.2 | 6.3 | ||||||
| [71] | CYN/corn straw (70:30 w:w) | HS 100 d | 16.3 | 13.8 | 24.7 | 28.9 | 5.6 | 10.6 | C10 | 34.7 | 11.5 | 30.7 | 10.4 | 3.1 | 9.5 | ||
| [88] | Coffee husks/lettuce residues at (60:40 w:w) | CT 100 d | 26.9 | 11.9 | 26.4 | 16.6 | 4.3 | 14 | C11 | 30.2 | 11.3 | 33.6 | 12.0 | 5.3 | 7.7 | ||
| CYN with maize straw/WC (70:30 w:w) | CT 100 d | 27.6 | 12.1 | 27.3 | 15.0 | 4.2 | 13.9 | [43] | SM/ rice straw (4:1 | ||||||||
| PEP with maize straw/WC (70:30 w:w | w:w) | FS | 24.6 | 57.9 | 7.7 | 9.8 | |||||||||||
| ) | CT 100 d | 17.4 | 14.3 | 31.1 | 19.2 | 5.8 | 12.3 | C 40 d | 23.2 | 55.2 | 9.8 | 11.8 | |||||
| [68] | Agricultural crop plants/NH4NO3 (66:34 w:w) | HA 90 d | 41.63 | 24.89 | 19.31 | 14.16 | SM/ rice straw (8:1 w:w) | FS | 28.8 | 50.5 | 7.9 | ||||||
| Date palm fronds/NH4NO3 (66:34 w:w | 12.8 | ||||||||||||||||
| ) | HA 90 d | 36.39 | 29.05 | 23.85 | C 40 d | 25.6 | 48.9 | 10.6 | 15.0 | ||||||||
| 10.70 | |||||||||||||||||
| Animal waste/NH4NO3 (66:34 w:w) | HA 90 d | 31.39 | 29.0 | SM/rice straw/biochar (8:1:1 w:w) | FS | 24.7 | 40.9 | 24.7 | 9.8 | ||||||||
| 25.45 | 14.33 | ||||||||||||||||
| [89] | Tomato R/escarole R/WC/CS (17.5:20.5:60:2) | CT 105 d | 23.50 | 15.60 | 28.65 | 16.80 | 5.25 | 10.20 | C 40 d | 19.2 | 28.8 | 42.5 | 9.5 | ||||
| Tomato R/escarole R/WC/CS (37:11:50:2) | CT 105 d | 22.55 | 14.95 | 26.65 | 18.25 | 5.90 | 11.75 | [10] | COF/fresh grass/mature compost (18:80:2 w:w) | C 6 d | 33.5 | 15.2 | 23.3 | 14.8 | 3.2 | 10 | |
| Tomato R/escarole R/WC/CS (50:0:48:2) | CT 105 d | 23.40 | 14.70 | 26.80 | 17.10 | 6.90 | 11.15 | [45] | Solid pig slurry/cotton gin waste (4:3 v:v) | FS | 13.5 | 5.9 | |||||
| Commercial compost from biowaste | 74.9 | CT 105 d | 26.95 | 12.85 | 3.7 | 0.9 | 1.1 | ||||||||||
| 32.70 | 10.35 | 2.90 | 14.25 | BT | 17.5 | 8.2 | 62.7 | 5.3 | 1.8 | 4.5 | |||||||
| [83] | DOW/COF/pine needles and WT (1:1:1) | DOM FS | 26.0 | 6.3 | AT | 26.0 | 12.3 | 51.2 | 4.2 | 2.5 | 3.9 | ||||||
| EB | 30.1 | 10.5 | 48.1 | 4.4 | 1.9 | 5.0 | |||||||||||
| AM | 35.5 | 13.1 | 43.8 | 3.6 | 0.7 | 3.4 | |||||||||||
| Solid pig slurry/cotton gin waste (3:4 v:v) | FS | 19.4 | 9.3 | 71.6 | 0.3 | 0.0 | 0.0 | ||||||||||
| BT | 13.6 | 7.1 | 71.6 | 5.5 | 1.0 | 1.2 | |||||||||||
| AT | 18.7 | 4.7 | 61.9 | 7.4 | 3.2 | 4.1 | |||||||||||
| EB | 26.4 | 11.4 | 53.8 | 5.7 | 4.5 | 6.8 | |||||||||||
| AM | 34.1 | 12.5 | 44.3 | 3.8 | 1.3 | 4.1 | |||||||||||
| Solid pig slurry/cotton gin waste (3:7 v:v) | FS | 22.9 | 7.4 | 63.6 | 2.3 | 1.1 | 2.7 | ||||||||||
| BT | 24.9 | 9.3 | 59.1 | 0.9 | 2.0 | 3.9 | |||||||||||
| AT | 21.0 | 9.3 | 65.5 | 1.1 | 0.9 | 2.2 | |||||||||||
| 42.1 | 9.5 | 4.9 | 11.1 | ||||||||||||||
| DOM 90 d | 30.8 | 7.4 | 27.1 | 13.4 | EB | 18.4 | 8.1 | 69.7 | 2.1 | 1.4 | 0.3 | ||||||
| AM | 21.8 | 9.0 | 58.8 | 2.6 | 2.6 | 5.2 | |||||||||||
| [51] | BM/CM/maize straw/PT (70:30 w:w) | FS | 15.4 | 10.5 | 58.4 | 8.6 | 3.0 | 4.1 | |||||||||
| C 108 d | 18.5 | 11.4 | 48.0 | 12.0 | 3.4 | 6.7 | |||||||||||
| +bioplastic (1 wt%) | C 108 d | 17.6 | 11.1 | 47.9 | 12.3 | 4.0 | 7.1 | ||||||||||
| +bioplastic (2 wt%) | C 108 d | 18.9 | 11.0 | 49.2 | 11.6 | 3.6 | 5.7 | ||||||||||
| 7.3 | [49] | SM/pumice | FS | 34.6 | 48.5 | 7.3 | 9.7 | ||||||||||
| C 10 d | 21.5 | 60.4 | 10.5 | 7.7 | |||||||||||||
| 14.0 | C 20 d | 23.6 | 57.7 | 10.9 | 7.7 | ||||||||||||
| C60 d | 26.2 | 48.3 | 12.9 | 12.7 | |||||||||||||
| CM/pumice | FS | 14.6 | 23.7 | 64.8 | 12.4 | 8.3 | |||||||||||
| 28.2 | |||||||||||||||||
| C 10 d | 9.0 | 70.4 | 12.9 | 7.7 | |||||||||||||
| C 20 d | 10. | 69.2 | 12.7 | 7.7 | |||||||||||||
| C60 d | 11.7 | 66.6 | 13.3 | 8.5 | |||||||||||||
| ChM/pumice | FS | 29.5 | 52.3 | 8.2 | 10.1 | ||||||||||||
| 20.3 | 27.8 | ||||||||||||||||
| HS 120 d | 25.4 | 34.9 | 16.4 | 23.3 | |||||||||||||
| [79] | MSW/vegetal wastes (1:1 v:v) | HA FS | 43.4 | 25.9 | 10.3 | 16.4 | 4.0 | ||||||||||
| HA 28 d | 44.7 | 22.1 | 11.0 | 17.9 | 4.3 | ||||||||||||
| HA 100 d | 42.9 | 20.3 | 11.4 | 17.9 | 7.5 | ||||||||||||
| [61 | C 10 d | 21.5 | ] | MSW | HA FS | 66.0 | 32 | 6.4 | 45 | 6.1 | |||||||
| 13 | 10 | C 20 d | 21.7 | 61.6 | 9.3 | 7.4 | |||||||||||
| HA 49 d | 48 | 26 | 16 | 10 | C60 d | 26.8 | 52.0 | 10.5 | 10.7 | ||||||||
| [82] | MSW | HA 6 d | 38 | 31 | 13 | 5 | 11 | 2 | |||||||||
| HA 19 d | 45 | 23 | 15 | 5 | 11 | 1 | |||||||||||
| HA 33 d | 44 | 23 | 16 | 6 | 11 | 1 | |||||||||||
| HA 62 d | 42 | 24 | 15 | 6 | 12 | 2 | |||||||||||
| HA 105 d | 39 | 25 | 16 | 6 | 12 | 2 | |||||||||||
| HA 187 d | 38 | 26 | 17 | 6 | 12 | 3 | |||||||||||
| Core-HA 6 d | 34 | 24 | 21 | 8 | 12 | 2 | |||||||||||
| Core-HA 19 d | 33 | 23 | 23 | 9 | 11 | 2 | |||||||||||
| Core-HA 33 d | 34 | 23 | 23 | 9 | 11 | 1 | |||||||||||
| Core-HA 62 d | 38 | 23 | 19 | 7 | 11 | 3 | |||||||||||
| Core-HA 105 d | 43 | 21 | 19 | 7 | 10 | 0 | |||||||||||
| Core-HA 187 d | 35 | 22 | 20 | 8 | 13 | 3 | |||||||||||
| [ | |||||||||||||||||
| 62 | |||||||||||||||||
| DOW/GT/FR (2:1:1) | DOM FS | 31.8 | 6.2 | 38.5 | 7.9 | 4.3 | 11.4 | ||||||||||
| DOM 90 d | 34.6 | 7.3 | 25.9 | 13.1 | 4.9 | 14.2 | |||||||||||
| GT/COF/spent yeast (1:1:1) | DOM FS | 30.8 | 5.3 | 41.2 | 6.2 | 2.8 | 13.6 | ||||||||||
| DOM 90 d | 35.2 | 8.4 | 22.7 | 11.8 | 5.8 | 16.2 | |||||||||||
| GT/COF/FR/sewage sludge (4:2:2.5:0.25) | DOM FS | 30.8 | 6.2 | 38.2 | 8.6 | 4.9 | 11.3 | ||||||||||
| DO 90d | 33.9 | 7.8 | 25.0 | 13.2 | 5.4 | 14.7 | |||||||||||
| [78] | DOW/GT/vegetal R from tobacco (50:30:20) | HA 60 d | 28.0 | 11.3 | 32.4 | 16.8 | 11.5 | ||||||||||
| HA 90 d | 34.9 | 10.8 | 28.6 | 15.7 | 10.0 | ||||||||||||
| HA 150 d | 34.5 | 9.3 | 23.1 | 19.8 | 13.4 | ||||||||||||
| [85] | OFMSW/GT/foliage R from tobacco (55:30:15) | CT 120 d | 31.0 | 9.0 | 23.1 | 13.3 | 23.6 | ||||||||||
| HoDOM 120 d | 34.6 | 12.6 | 19.5 | 19.3 | 14.0 | ||||||||||||
| HiDOM 120 d | 30.3 | 9.8 | 36.8 | 9.1 | 14.0 | ||||||||||||
| [70] | OvM/straw | HS FS | 18.6 | 49.7 | 8.2 | 23.5 | |||||||||||
| HS 120 d | 17.7 | 25.0 | 22.3 | 35.0 | |||||||||||||
| Mixture of animal manures | HS FS | 33.7 | 13.8 | 11.8 | 40.7 | [50] | Exhausted grape marc/CM (76:24 w:w) | FS | 30.7 | 17.0 | 39.5 | 3.3 | 3.5 | 6.1 | |||
| C 28 d | 36.4 | 15.9 | 31.2 | 5.7 | 3.7 | 7.3 | |||||||||||
| C105 d | 37.9 | 16.1 | 28.9 | 6.3 | 3.9 | 6.9 | |||||||||||
| C 168 d | 37.3 | 14.3 | 22.4 | 7.1 | 4.8 | 14.2 | |||||||||||
| Grape marc/CM (72:28 w:w) | FS | 33.6 | 15.3 | 37.1 | 2.9 | 3.7 | 7.4 | ||||||||||
| C 28 d | 34.3 | 13.7 | 37.3 | 3.6 | 3.6 | 7.5 | |||||||||||
| C105 d | 33.3 | 14.8 | 38.6 | 3.8 | 3.0 | 6.6 | |||||||||||
| C 168 d | 34.9 | 15.2 | 34.4 | 3.3 | 4.0 | 8.2 | |||||||||||
| Exhausted grape marc/PM (67:33 w:w) | FS | 30.8 | 14.2 | 41.0 | 3.5 | 3.5 | 7.0 | ||||||||||
| C 28 d | 34.4 | 14.6 | 35.4 | 3.2 | 4.0 | 8.5 | |||||||||||
| C105 d | 33.9 | 15.5 | 35.7 | 3.7 | 3.9 | 7.5 | |||||||||||
| C 168 d | 34.3 | 15.5 | 31.5 | 3.4 | 5.0 | 10.2 | |||||||||||
| [42] | CM | CC | |||||||||||||||
| HS 120 d | 30.4 | 24.0 | 9.6 | 36.0 | |||||||||||||
| Solid olive mill wastes | HS FS | 23.2 | 56.4 | 11.2 | 9.2 | ||||||||||||
| HS 120 d | 18.2 | 7.6 | 48.8 | 8.9 | 5.0 | 11.6 | |||||||||||
| Broiler litter | CC | 17.9 | 9.1 | 48 | 8.0 | 5.2 | 11.7 | ||||||||||
| Green waste | CC | 22.4 | 10.7 | 40.4 | 8.2 | 6.3 | 12.0 | ||||||||||
| Nitro-humus | CC | 19.6 | 8.8 | 43 | 9.6 | 6.7 | 12.4 | ||||||||||
| MSW | CC | 23.0 | 7.6 | 48.7 | 7.3 | 3.6 | 9.8 | ||||||||||
| [56] | Rice husk/rice bran/BEM/molasses | FS | 2.15 | 25.8 | 67.4 | 2.98 | 0.99 | ||||||||||
| 22.2 | 29.3 | C 13 d | 1.98 | 25.6 | 68.9 | 3.07 | 0.51 | ||||||||||
| 19.7 | C 34 d | 0.66 | 25.6 | 70.8 | 2.98 | - | |||||||||||
| C 53 d | 1.10 | 22.6 | 73.1 | 3.29 | - | ||||||||||||
| C 61 d | 1.03 | 24.0 | 71.9 | 3.09 | - | ||||||||||||
| C 116 d | 0.88 | 25.7 | 69.9 | 2.63 | 0.88 | ||||||||||||
| [55] | DOW/plant trimming/vegetal R (50:40:10 w:w) | C 60 d | 37.6 | 50.8 | 7.2 | 4.3 | |||||||||||
| C 90 d | 30.6 | 56.1 | 7.3 | 6.1 | |||||||||||||
| C 150 d | 45.3 | 37.6 | 9.6 | 7.4 | |||||||||||||
| [46] | CM/rice straw | FS | 14.9 | 54.9 | 20.3 | 10.0 | |||||||||||
| 28.8 | |||||||||||||||||
| Solid wastes of wineries | HS FS | 3.5 | 46.9 | 1.5 | 48.1 | ||||||||||||
| HS 120 d | 21.6 | 13.1 | 26.8 | 38.5 | |||||||||||||
| Domestic wastes | HS FS | ||||||||||||||||
| [81] | OFMSW | HA FS | 37.20 | 34.34 | 16.28 | 12.23 | C 60 d | 17.4 | 57.4 | 20.9 | 10.6 | ||||||
| C 120 d | |||||||||||||||||
| HA C | 30.07 | 34.71 | 22.67 | 12.58 | 17.4 | 48.6 | 18.6 | 9.9 | |||||||||
| C 240 d | 15.2 | 34.1 | 16.1 | 9.2 | |||||||||||||
| C 365 d | 13.9 | 25.7 | 12.4 | 6.5 | |||||||||||||
| C 548 d | 12.8 | 22.8 | 11.3 | 6.6 | |||||||||||||
| FS | 13.5 | 56.7 | 20.1 | 9.7 | |||||||||||||
| C 148 d | 15.2 | 39.6 | 18.7 | 10.0 | |||||||||||||
| FS | 15.2 | 56.4 | 18.8 | 9.6 | |||||||||||||
| C 60 d | 13.4 | 47.5 | 16.5 | 8.4 | |||||||||||||
| C 120 d | 12.6 | 42.0 | 17.5 | 8.5 | |||||||||||||
| C 240 d | 12.1 | 37.2 | 15.9 | 8.1 | |||||||||||||
| C 365 d | 12.6 | 35.6 | 15.3 | 7.7 | |||||||||||||
| [44] | SM/wheat straw (95:5 w:w) | FS | 27.2 | 55.6 | 9.2 | 8.0 | |||||||||||
| C 7 d | 18.6 | 66.1 | 9.8 | 5.5 | |||||||||||||
| C 14 d | 15.2 | 65.3 | 12.2 | 7.3 | |||||||||||||
| C 21 d | 16.8 | 65.2 | 11.5 | 6.5 | |||||||||||||
| C 28 d | 14.7 | 63.3 | 13.6 | 8.3 | |||||||||||||
| [61] | MSW (composted in spring) | FS | 15.8 | 59.5 | 14.9 | 7.9 | |||||||||||
| C 28 d | 17.7 | 59.2 | 13.6 | 7.4 | |||||||||||||
| C 42 d | 17.5 | 55.5 | 16.2 | 7.2 | |||||||||||||
| C 49 d | 17.3 | 55.5 | 17.7 | 5.4 | |||||||||||||
| MSW (composted in summer) | FS | 16.2 | 60.4 | 12.1 | 7.8 | ||||||||||||
| C 28 d | 16.8 | 60.2 | 13.9 | 6.9 | |||||||||||||
| C 42 d | 18.0 | 56.1 | 15.3 | 6.8 | |||||||||||||
| C 49 d | 18.2 | 56.9 | 15.8 | 6.0 | |||||||||||||
| [60] | Kitchen waste/garden waste | C1 | 28.4 | 45.6 | 7.4 | 4.6 | 14.0 | ||||||||||
| C2 | 25.4 | 48.6 | 9.8 | 4.8 | 11.4 | ||||||||||||
| C3 | 30.3 | 32.5 | 11.1 | 6.3 | 19.8 | ||||||||||||
| C4 | 32.4 | 38.2 | 7.9 | 4.9 | 16.6 | ||||||||||||
| C5 | 19.2 | 53.0 | 12.8 | 8.2 | 6.8 | ||||||||||||
| C6 | 25.5 | 40.8 | 12.6 | 6.6 | 14.5 | ||||||||||||
| C7 | 27.4 | 42.8 | 11.3 | 6.3 | 12.2 | ||||||||||||
| C8 | 26.7 | 42.6 | 9.0 | 7.9 | 13.8 | ||||||||||||
| C9 | 14.1 | 43.6 | 8.1 | 12.0 | ] | MSW | FS | 26.9 | 47.9 | 11.6 | 4.3 | 8.3 | |||||
| C 34 d | 25.5 | 52.1 | 10.5 | 3.6 | 8.2 | ||||||||||||
| C 76 d | 24.7 | 46.5 | 13.6 | 4.9 | 10.3 | ||||||||||||
| C 90 d | 23.6 | 42.4 | 19.0 | 6.6 | 11.4 | ||||||||||||
| C132 d | 23.6 | 40.4 | 16.9 | 7.6 | 11.4 | ||||||||||||
| [59] | Grape skin | FS | 8.7 | 53.4 | 17.5 | 18.7 | |||||||||||
| C 160 d | 10.9 | 51.8 | 18.9 | 17.0 | |||||||||||||
| Grape seeds | FS | 25.0 | 41.3 | 13.1 | 18.7 | ||||||||||||
| C 160 d | 21.7 | 38.4 | 15.7 | 22.3 | |||||||||||||
| Grape skin and seeds | FS | 16.2 | 49.7 | 13.2 | 18.8 | ||||||||||||
| C 160 d | 18.6 | 43.7 | 15.9 | 20.3 | |||||||||||||
4. Conclusions and Perspectives
13C SSNMR, widely used in the field of materials science, has revealed as a powerful tool also for the investigation of organic natural matter of relevance in agriculture and environmental science. In fact, 13C SSNMR gives access to information on the composition of complex organic materials that cannot be obtained otherwise and which can be of great relevance for the optimization of waste recycling processes and the exploitation of waste materials that presently are directly disposed of in landfills.
13C SSNMR has been applied to investigate the composting of materials of varied nature, from relatively homogeneous garden wastes to widely heterogenous municipal solid wastes. Most studies have shown that the compositional features of compost depend on the starting feedstock and this is important for the application of these materials, which are often intended to be used as fertilizers or amendments in agricultural soils. Nevertheless, 13C SSNMR has highlighted the degradation of labile OM components (mainly carbohydrates) and the preservation of the recalcitrant ones (lipids, waxes, lignin). The relative amounts of the different components obtained from NMR data have been correlated with stability and maturity indices determined by analytical methods. In some cases, the results indicate that waste pretreatments may be required, as for example in the case of municipal solid waste where non-biodegradable plastic materials remain in the compost.
Although not conclusive alone, 13C SSNMR has contributed to the understanding of structure-bioactivity relationship of composts, highlighting the role of hydrophobic and polar chemical functionalities in determining the biostimulation properties of compost of interest in agriculture and the antibacterial, antiflammatory and antioxidant properties of extracts of relevance for their application in pharmacology.
Most of the studies cited relied on the spectral analysis based on the division of the 13C MAS NMR spectra in regions related to specific functional groups. This is certainly useful for understanding organic matter evolution during composting. However, the results of the different studies indicate that not always the standard decomposition pathway is followed and no univocal indices of humification and maturity, as those typically used in soil science, can be defined. This is probably due to the variable and complex composition of waste materials, especially in the case of municipal solid waste. Nonetheless, comparison of 13C MAS NMR spectra before and after the composting process yields detailed information on the transformations that have occurred, which is important for process optimization. To this end, molecular models can be of help, especially for the characterization and quality assessment of the final products, and would certainly deserve more consideration and possibly further refinement. Moreover, given the complex nature of OM in waste transformation, a combined 13C SSNMR investigation of the whole sample and different extracts is advisable for a better comprehension of the degradation processes and the chemical structure of the final products.
As evidenced by the growing literature in the field and by the results therein reported, 13C SSNMR is an important instrument for the investigation of organic waste transformation within a circular economy model. It can be easily foreseen that research in this field will experience a significant growth given the increased public awareness of the importance of waste recycling, and that 13C SSNMR will become even more important in the future in this field thanks to the rapid hardware and software advancements that NMR technology is experiencing, which will extend the information that can be obtained on complex materials as those considered in this review.References
- Bandini, F.; Taskin, E.; Bellotti, G.; Vaccari, F.; Misci, C.; Guerrieri, M.C.; Cocconcelli, P.S.; Puglisi, E. The treatment of the organic fraction of municipal solid waste (OFMSW) as a possible source of micro- and nano-plastics and bioplastics in agroecosystems: A review. Chem. Biol. Technol. Agric. 2022, 9, 4.
- Available online: https://datatopics.worldbank.org/what-a-waste/trends_in_solid_waste_management.html (accessed on 20 January 2023).
- Tejaswini, M.S.S.R.; Pathak, P.; Gupta, D.K. Sustainable approach for valorization of solid wastes as a secondary resource through urban mining. J. Environ. Manag. 2022, 319, 115727.
- Coma, M.; Martinez-Hernandez, E.; Abeln, F.; Raikova, S.; Donnelly, J.; Arnot, T.C.; Allen, M.J.; Hong, D.D.; Chuck, C.J. Organic waste as a sustainable feedstock for platform chemicals. Faraday Discuss. 2017, 202, 175–195.
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. Biotechnol. 2017, 16, 81–130.
- Shah, V.; Singh, A.; Mohanty, S.S.; Srivastava, V.K.; Varjani, S. Organic solid waste: Biorefinery approach as a sustainable strategy in circular bioeconomy. Bioresour. Technol. 2022, 349, 126835.
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energy Rev. 2018, 88, 326–346.
- Kumar Khanal, S.; Lü, F.; Wong, J.W.C.; Wu, D.; Oechsner, H. Anaerobic digestion beyond biogas. Bioresour. Technol. 2021, 337, 125378.
- Albrecht, R.; Ziarelli, F.; Alarcón-Gutiérrez, E.; Le Petit, J.; Terrom, G.; Perissol, C. 13C solid-state NMR assessment of decomposition pattern during co-composting of sewage sludge and green wastes. Eur. J. Soil Sci. 2008, 59, 445–452.
- Papale, M.; Romano, I.; Finore, I.; Lo Giudice, A.; Piccolo, A.; Cangemi, S.; Di Meo, V.; Nicolaus, B.; Poli, A. Prokaryotic diversity of the composting thermophilic phase: The case of ground coffee compost. Microorganisms 2021, 9, 218.
- Jara-Samaniego, J.; Perez-Murcia, M.D.; Bustamante, M.A.; Perez-Espinosa, A.; Paredes, C.; Lopez, M.; López-Lluch, D.B.; Gavilanes-Teráne, I.; Moral, R. Composting as sustainable strategy for municipal solid waste management in the Chimborazo Region, Ecuador: Suitability of the obtained composts for seedling production. J. Clean. Prod. 2017, 141, 1349–1358.
- Cesaro, A.; Conte, A.; Belgiorno, V.; Siciliano, A.; Guida, M. The evolution of compost stability and maturity during the full-scale treatment of the organic fraction of municipal solid waste. J. Environ. Manag. 2019, 232, 264–270.
- Palaniveloo, K.; Amran, M.A.; Norhashim, N.A.; Mohamad-Fauzi, N.; Peng-Hui, F.; Hui-Wen, L.; Kai-Lin, Y.; Jiale, L.; Chian-Yee, M.G.; Jing-Yi, L.; et al. Food waste composting and microbial community structure profiling. Processes 2020, 8, 723.
- Available online: https://www.compostnetwork.info/wordpress/wp-content/uploads/ECN-rapport-2022.pdf (accessed on 20 January 2023).
- Wu, L.; Ma, L.Q.; Martinez, G.A. Comparison of methods for evaluating stability and maturity of biosolids compost. J. Environ. Qual. 2000, 29, 424–429.
- Ouatmane, A.; Provenzano, M.R.; Hafidi, M.; Senesi, N. Compost maturity assessment using calorimetry, spectroscopy and chemical analysis. Compost Sci. Util. 2000, 8, 124–134.
- Butler, T.A.; Sikora, L.J.; Steinhilber, P.M.; Douglass, L.W. Compost age and sample storage effects on maturity indicators of biosolids compost. J. Environ. Qual. 2001, 30, 2141–2148.
- Chen, Y. Nuclear magnetic resonance, infra-red and pyrolysis: Application of spectroscopic methodologies to maturity determination of composts. Compost Sci. Util. 2003, 11, 152–168.
- Francou, C.; Poitrenaud, M.; Houot, S. Stabilization of organic matter during composting: Influence of process and feedstocks. Compost Sci. Util. 2005, 13, 72–83.
- Wichuk, K.M.; McCartney, D. Compost stability and maturity evaluation—A literature review. Can. J. Civ. Eng. 2010, 37, 1505–1523.
- Oviedo-Ocaña, E.R.; Torres-Lozada, P.; Marmolejo-Rebellon, L.F.; Hoyos, L.V.; Gonzales, S.; Barrena, R.; Komilis, D.; Sanchez, A. Stability and maturity of biowaste composts derived by small municipalities: Correlation among physical, chemical and biological indices. Waste Manag. 2015, 44, 63–71.
- Simpson, A.J.; Simpson, M.J. Nuclear magnetic resonance analysis of natural organic matter. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 589–651.
- Simpson, A.J.; McNally, D.J.; Simpson, M.J. NMR spectroscopy in environmental research: From molecular interactions to global processes. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 58, 97–175.
- Baldock, J.A.; Oades, J.M.; Nelson, P.N.; Skene, T.M.; Golchin, A.; Clarke, P. Assessing the extent of decomposition of natural organic materials using solid-state 13C NMR spectroscopy. Aust. J. Soil Res. 1997, 35, 1061–1083.
- Meija, J.; Coplen, T. B.; Berglund, M.; Brand, W. A.; De Bièvre, P.; Gröning, M.; Holden, N. E.; Irrgeher, J.; Loss, R. D.; Walczyk, T.; Prohaska, T. Isotopic compositions of the elements 2013 (IUPAC technical report). Pure Appl. Chem. 2016, 88, 293-306.
- Holden, N. E.; Coplen, T. B.; Böhlke, J. K.; tarbox, L. V.; Benefield, J.; de Laeter, J. R.; Mahaffy, P. G.; O’Connorr, G.; Roth, E.; Tepper, D. H.; Walczyk, T.; Wieser, M. E.; Yoneda, S. IUPAC periodic table of the elements and isotopes (IPTEI) for the educa-tion community (IUPAC technical report). Pure Appl. Chem. 2018, 90, 1833-2092.
- Pichler, M.; Knicker, H.; Kögel-Knabner, I. Changes in the chemical structure of municipal solid waste during composting as studied by solid-state dipolar dephasing and PSRE 13C NMR and solid-state 15N NMR spectroscopy. Environ. Sci. Technol. 2000, 34, 4034–4038.
- Réveillé, V.; Mansuy, L.; Jardé, E.; Garnier-Sillam, E. Characterization of sewage–sludge derived organic matter: lipids and humic acids. Org. Geochem. 2003, 34, 615–627.
- Pereira, M. A.; Pires, O. C.; Mota, M.; Alves, M. M. Anaerobic biodegradation of oleic and palmitic acids: evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol. Bioeng. 2005, 92, 15-23.
- Dignac, M. F.; Derenne, S.; Ginestet, P.; Bruchet, A.; Kniker, H.; Largeau, C. Determination of structure and origin of refractory organic matter in biodepurated wastewater via spectroscopic methods. Comparison of conventional and ozonation treat-ment. Environ. Sci. Technol. 2000, 34, 3389–3394.
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139-162.
- Ussiri, A. A. N.; Johnson, C. E. Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectros-copy and chemicals methods. Geoderma 2003, 11, 123–149.
- Atalla, R. H.; VanderHart, D. L. The role of solid state 13C NMR spectroscopy in studies of the nature of native cellulose. Solid State Nucl. Magn. Reson. 1999, 15, 1−19.
- Hatcher, P. G. Chemical structural studies of natural lignin by dipolar dephasing solid-state 13C nuclear magnetic resonance. Org. Geochem. 1987, 11, 31−39.
- Manu, M. K.; Kumar, R.; Garg, A. Performance assessment of improved composting system for food waste with varying aer-ation and use of microbial inoculum. Bioresource Technol. 2017, 234, 167-177.
- Bustamante, M. A.; Paredes, C.; Marhuenda-Egea, F. C.; Perez-Espinosa, A.; Bernal, M. P.; Moral, R. Co-composting of distill-ery wastes with animal manures: carbon and nitrogen transformations in the evaluation of compost stability. Chemosphere 2008, 72, 551–557.
- Zhou, Y.; Selvam, A.; Wong, J. W. C. Evaluation of humic substances during co-composting of food waste, sawdust and Chi-nese medicinal herbal residues, Bioresource Technol. 2014, 168, 229-234.
- Chefetz, B.; Hadar, Y.; Chen, Y. Dissolved organic carbon fractions formed during composting of municipal solid waste: properties and significance. Acta Hydrochim. Hydrobiol. 1998, 26, 172-179.
- Eudoxie, G.; Martin, M. Compost tea quality and fertility, In Organic fertilizers: history, production and applications, Lar-ramendy, M., Soloneski, S., Eds. IntechOpen: London, UK, 2019.
- Romero, C.M.; Redman, A.-A.P.H.; Owens, J.; Terry, S.A.; Ribeiro, G.O.; Gorzelak, M.A.; Oldenburg, T.B.P.; Hazendonk, P.; Larney, F.J.; Hao, X.; et al. Effects of feeding a pine-based biochar to beef cattle on subsequent manure nutrients, organic matter composition and greenhouse gas emissions. Sci. Total Environ 2022, 812, 152267.
- Iacomino, G.; Sarker, T.C.; Ippolito, F.; Bonanomi, G.; Vinale, F.; Staropoli, A.; Idbella, M. Biochar and compost application either alone or in combination affects vegetable yield in a volcanic Mediterranean soil. Agronomy 2022, 12, 1996.
- Eldridge, S.M.; Chen, C.R.; Xu, Z.H.; Nelson, P.N.; Boyd, S.E.; Meszaros, I.; Chan, K.Y. Molecular composition of recycled organic wastes, as determined by solid-state 13C NMR and elemental analyses. Waste Manag. 2013, 33, 2157–2169.
- Liu, H.; Guo, H.; Guo, X.; Wu, S. Probing changes in humus chemical characteristics in response to biochar addition and varying bulking agents during composting: A holistic multi-evidence-based approach. J. Environ. Manag. 2021, 300, 113736.
- Veeken, A.H.M.; Adani, F.; Nierop, K.G.J.; de Jager, P.A.; Hamelers, H.V.M. Degradation of biomacromolecules during high-rate composting of wheat straw–amended feces. J. Environ. Qual. 2001, 30, 1675–1684.
- Martín-Mata, J.; Lahoz-Ramos, C.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Moral, R.; Santos, A.; Sáez, J.A.; Bernal, M.P. Thermal and spectroscopic analysis of organic matter degradation and humification during composting of pig slurry in different scenarios. Environ. Sci. Pollut. Res. 2016, 23, 17357–17369.
- Tang, J.-C.; Maie, N.; Tada, Y.; Katayama, A. Characterization of the maturing process of cattle manure compost. Process Biochem. 2006, 41, 380–389.
- Inbar, Y.; Chen, Y.; Hadar, Y. Solid-state carbon-13 nuclear magnetic resonance and infrared spectroscopy of composted organic matter. Soil Sci. Soc. Am. J. 1989, 53, 1695–1701.
- Chen, Y.; Inbar, Y.; Hadar, Y.; Malcolm, R.L. Chemical properties and solid-state CPMAS 13C-NMR of composted organic matter. Sci. Total Environ. 1989, 81–82, 201–208.
- Wang, K.; He, C.; You, S.; Liu, W.; Wang, W.; Zhang, R.; Qi, H.; Ren, N. Transformation of organic matters in animal wastes during composting. J. Hazard. Mater. 2015, 300, 745–753.
- Torres-Climent, A.; Gomis, P.; Martín-Mata, J.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Paredes, C.; Moral, R. Chemical, thermal and spectroscopic methods to assess biodegradation of winery-distillery wastes during composting. PLoS ONE 2015, 10, e0138925.
- Spaccini, R.; Todisco, D.; Drosos, M.; Nebbioso, A.; Piccolo, A. Decomposition of bio-degradable plastic polymer in a real on-farm composting process. Chem. Biol. Technol. Agric. 2016, 3, 4.
- Lin, Y.-H.; Lin, Y.-Z.; Lin, Y.-H. Preliminary design for establishing compost maturity by using the spectral characteristics of five organic fertilizers. Sci. Rep. 2022, 12, 15721.
- Spaccini, R.; Piccolo, A. Spectroscopic characterization of compost at different maturity stages. Clean 2008, 36, 152–157.
- Knicker, H.; Lüdemann, H.-D. N-15 and C-13 CPMAS and solution NMR studies of N-15 enriched plant material during 600 days of microbial degradation. Org. Geochem. 1995, 23, 329–341.
- Spaccini, R.; Piccolo, A. Molecular characterization of compost at increasing stages of maturity. 2. Thermochemolysis-GC-MS and 13C-CPMAS-NMR spectroscopy. J. Agric. Food Chem. 2007, 55, 2303–2311.
- Anda, M.; Syed Omar, S.R.; Shamshuddin, J.; Fauziah, C.I. Changes in properties of composting rice husk and their effects on soil and cocoa growth. Commun. Soil Sci. Plant Anal. 2008, 39, 2221–2249.
- Caricasole, P.; Provenzano, M.R.; Hatcher, P.G.; Senesi, N. Evolution of organic matter during composting of different organic wastes assessed by CPMAS 13C NMR spectroscopy. Waste Manag. 2011, 31, 411–415.
- Pane, C.; Spaccini, R.; Caputo, M.; De Falco, E.; Zaccardelli, M. Multi-parameter characterization of disease-suppressive bio-composts from aromatic plant residues evaluated for garden cress (Lepidium sativum L.) cultivation. Horticulturae 2022, 8, 632.
- Inbar, Y.; Chen, Y.; Hadar, Y. Carbon-13 CPMAS NMR and FTIR spectroscopic analysis of organic matter transformations during composting of solid wastes from wineries. Soil Sci. 1991, 152, 272–282.
- Preston, C.M.; Cade-Menun, B.J.; Sayer, B.G. Characterization of Canadian backyard composts: Chemical and spectroscopic analyses. Compost Sci. Util. 1998, 6, 53–66.
- González-Vila, F.J.; Almendros, G.; Madrid, F. Molecular alterations of organic fractions from urban waste in the course of composting and their further transformation in amended soil. Sci. Total Environ. 1999, 236, 215–229.
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y. Chemical and biological characterization of organic matter during composting of municipal solid waste. J. Environ. Qual. 1996, 25, 776–785.
- Nelson, P.N.; Baldock, J.A. Estimating the molecular composition of a diverse range of natural organic materials from solid-state 13C NMR and elemental analyses. Biogeochemistry 2005, 72, 1–34.
- Zmora-Nahum, S.; Hadar, Y.; Chen, Y. Physico-chemical properties of commercial composts varying in their source materials and country of origin. Soil Biol. Biochem. 2007, 39, 1263–1276.
- Haw, J.F.; Maciel, G.E.; Schroeder, H.A. Carbon-13 nuclear magnetic resonance spectrometric study of wood and wood pulping with cross polarization and magic-angle spinning. Anal. Chem. 1984, 56, 1323–1329.
- Huang, J.; Yu, Z.; Gao, H.; Yan, X.; Chang, J.; Wang, C.; Hu, J.; Zhang, L. Chemical structures and characteristics of animal manures and composts during composting and assessment of maturity indices. PLoS ONE 2017, 12, e0178110.
- Skene, T.M.; Skjemstad, J.O.; Oades, J.M.; Clarke, P.J. The influence of inorganic matrices on the decomposition of straw. Aust. J. Soil Res. 1996, 34, 413–426.
- Al-Faiyz, Y.S.S. CPMAS 13C NMR characterization of humic acids from composted agricultural Saudi waste. Arab. J. Chem. 2017, 10, 5839–5853.
- Verrillo, M.; Salzano, M.; Savy, D.; di Meo, V.; Valentini, M.; Cozzolino, V.; Piccolo, A. Antibacterial and antioxidant properties of humic substances from composted agricultural biomasses. Chem. Biol. Technol. Agric. 2022, 9, 28.
- Fuentes, M.; Baigorri, R.; González-Gaitano, G.; García-Mina, J.M. The complementary use of 1H NMR, 13C NMR, FTIR and size exclusion chromatography to investigate the principal structural changes associated with composting of organic materials with diverse origin. Org. Geochem. 2007, 38, 2012–2023.
- Verrillo, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Humic substances from green compost increase bioactivity and antibacterial properties of essential oils in basil leaves. Chem. Biol. Technol. Agric. 2021, 8, 28.
- Zheng, W.; Yang, Z.; Huang, L.; Chen, Y. Roles of organic matter transformation in the bioavailability of Cu and Zn during sepiolite-amended pig manure composting. J. Environ. Manag. 2022, 314, 115046.
- Guo, X.; Liu, H.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899.
- Ngo, P.; Rumpel, C.; Ngo, Q.; Alexis, M.; Vargas, G.V.; Mora Gil, M.D.L.L.; Dang, D.; Jouquet, P. Biological and chemical reactivity and phosphorus forms of buffalo manure compost, vermicompost and their mixture with biochar. Bioresour. Technol. 2013, 148, 401–407.
- Chen, Y.; Huang, X.; Han, Z.; Huang, X.; Hu, B.; Shi, D.; Wu, W. Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting. Chemosphere 2010, 78, 1177–1181.
- Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24.
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510.
- Spaccini, R.; Piccolo, A. Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil Biol. Biochem. 2009, 41, 1164–1172.
- Castaldi, P.; Alberti, G.; Merella, R.; Melis, P. Study of the organic matter evolution during municipal solid waste composting aimed at identifying suitable parameters for the evaluation of compost maturity. Waste Manag. 2005, 25, 209–213.
- Almendros, G.; Dorado, J.; González-Vila, F.J.; Blanco, M.J.; Lankes, U. 13C NMR assessment of decomposition patterns during composting of forest and shrub biomass. Soil Biol. Biochem. 2000, 32, 793–804.
- García, C.; Hernández, T.; Costa, F. Comparison of humic acids derived from city refuse with more developed humic acids. J. Soil Sci. Plant Nutr. 1992, 38, 339–346.
- Chefetz, B.; Adani, F.; Genevini, P.; Tambone, F.; Hadar, Y.; Chen, Y. Humic-acid transformation during composting of municipal solid waste. J. Environ. Qual. 1998, 27, 794–800.
- Caricasole, P.; Provenzano, M.R.; Hatcher, P.G.; Senesi, N. Chemical characteristics of dissolved organic matter during composting of different organic wastes assessed by 13C CPMAS NMR spectroscopy. Bioresour. Technol. 2010, 101, 8232–8236.
- Vinceslas-Akpa, M.; Loquet, M. Organic matter transformations in lignocellulosic waste products composted or vermicomposted (Eisenia Fetida Andrei): Chemical analysis and 13C CPMAS NMR spectroscopy. Soil Biol. Biochem. 1997, 29, 751–758.
- Spaccini, R.; Baiano, S.; Gigliotti, G.; Piccolo, A. Molecular characterization of a compost and its water-soluble fractions. J. Agric. Food Chem. 2008, 56, 1017–1024.
- Açıkgöz, M.A. Evaluation of phytochemical compositions and biological properties of Achillea gypsicola at different phenological stages. Chem. Biodivers. 2019, 16, e1900373.
- Verrillo, M.; Parisi, M.; Savy, D.; Caiazzo, G.; di Caprio, R.; Luciano, M.A.; Cacciapuoti, S.; Fabbrocini, G.; Piccolo, A. Antiflammatory activity and potential dermatological applications of characterized humic acids from a lignite and a green compost. Sci. Rep. 2022, 12, 2152.
- Verrillo, M.; Salzano, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Bioactivity and antimicrobial properties of chemically characterized compost teas from different green composts. Waste Manage. 2021, 120, 98–107.
- Pane, C.; Palese, A.M.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Enhancing sustainability of a processing tomato cultivation system by using bioactive compost teas. Sci. Hortic. 2016, 202, 117–124.
- Canellas, L.P.; Piccolo, A.; Dobbss, L.B.; Spaccini, R.; Olivares, F.L.; Zandonadi, D.B.; Façanha, A.R. Chemical composition and bioactivity properties of size-fractions separated from a vermicompost humic acid. Chemosphere 2010, 78, 457–466.
