Lipid nanoparticles (LNPs) have recently emerged as one of the most advanced technologies for the highly efficient in vivo delivery of exogenous mRNA, particularly for COVID-19 vaccine delivery. LNPs comprise four different lipids: ionizable lipids, helper or neutral lipids, cholesterol, and lipids attached to polyethylene glycol (PEG). Since its first isolation in 1961, mRNA (that encodes the protein of interest) research has taken several paths, which made us understand its diversified functions and modification-mediated potential for therapeutic applications. As a result of the COVID-19 pandemic, nucleic acid therapeutics (NATs), particularly mRNA vaccines, potentials have been enabled for emerging infectious diseases. The translation of host genetic information (DNA) into proteins by ribosomes in the cytoplasm is mediated by mRNA.
1. Use of mRNA as Prophylactics and Therapeutics
Despite the usage of DNA vaccines, RNA-based vaccines have grown in popularity in the 21st century. Interestingly, mRNA vaccine technology purely depends on the genetic code of the virus; since the SARS-CoV-2 genetic sequence was published
[1][58], virally generated self-amplifying RNA and non-replicating mRNA are being used abundantly for vaccine development. Indeed, during the COVID-19 pandemic, mRNA vaccines have become the only vaccine source available to combat the rapid spread of disease. While multiple pharmaceutical companies have investigated several vaccine technologies against COVID-19, the FDA has approved two vaccines (first for emergency use and then for full approval), Pfizer/BioNTech’s and Moderna’s Spikevax (
Table 12)
[2][59]. Both use the complete sequence of spike and receptor-binding domains of SARS-CoV-2 as target antigens. However, mRNA-based preclinical studies have been reported since 1990. Its poor stability as naked mRNA and tremendous capacity to induce an innate immune response (particularly double-stranded RNA) has halted the progress of RNA-based therapeutics. The emergence of advanced nanotechnologies for the protection and targeted delivery of water-soluble molecules have shined a light on developing the LNP-mRNA vaccines. Nucleic acid delivery to the cytosol, without hampering the cell wall integrity, and degradation by nucleases is a rate limiting step for targeted delivery. Despite mechanical and viral particle-mediated targeted delivery, chemical based targeted delivery via LNPs have been the ease of the process and have been less toxic, with less immunogenicity. During the start of the decade, several clinical trials have been initiated on mRNA therapeutics, which cover a range of different types of diseases, such as cancer, infectious diseases, protein replacement therapy, and others. Correspondingly, promising results have been shown in terms of disease alleviation, reduction of pathogenicity, and others. Interestingly, several diseases or disorders are targeted by using mRNA therapeutics. For the proof-of-concept studies, in vitro transcription (IVT)-produced mRNA is more suitable (
Figure 15). To characterize the efficiency of mRNA in vitro, several transfection reagents are available to facilitate mRNA translation and targeted protein production, although they are not as efficient as targeted delivery systems. The
Figure 15 shows the most successful mRNA vaccines. As explained earlier, a suitable (perfect mix) vehicle is needed for the mRNA vaccine delivery. LNPs have become the one of the most characterized mRNA delivery vehicles.
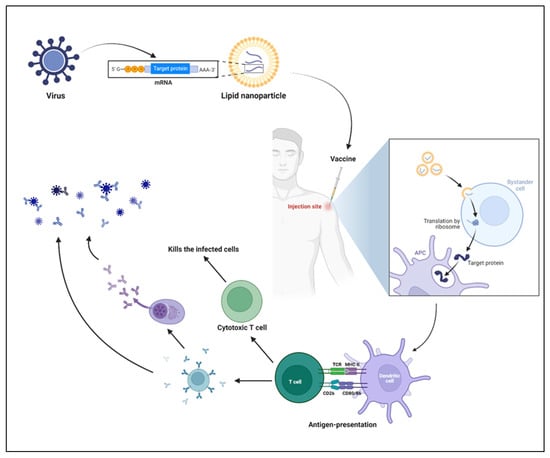
Figure 15. Immune response by mRNA vaccines. LNPs are prepared by encapsulating mRNA, which encodes the viral protein of interest. Upon injection of vaccines, muscular cells take up the LNPs following the release of mRNA into the cytosol and translation of target protein with the help of host machinery. In parallel, the danger associated signals produced by the LNPs recruit the innate immune cells, including neutrophils, monocytes, macrophages, dendritic cells, and others. The antigen-presenting cells (APC) process and present the antigen to the T cells, which further polarizes into effector T cells and helps in B cell-mediated responses. The cytotoxic T cells produced upon activation kill the infected cells, and antibodies (produced by B cells or plasma cells) neutralize the virus.
2. LNP Role in mRNA Delivery
Using lipid-encapsulated forms of sequence-optimized mRNA vaccines has shown strong immunity (both in preclinical and clinical) against infectious disease targets like the flu virus, Zika virus, rabies virus, and others, especially in the last few years
[3][60]. Of note, the LNPs act as potential vaccine adjuvants
[2][59], which helps in shaping antigen-specific immunity
[4][61]. Despite several components of the LNP, ionizable lipids have a prominent role, including forming LNPs and the targeted delivery of mRNA into the cytosol. The ionizable lipid imparts the neutral charge to the LNPs at physiological pH and ionizes at lower pH in the endosome/lysosome, followed by the delivery of intact mRNA to the cytoplasm, where it can then be translated into the encoded protein. Despite mRNA, LNPs also induce the innate immune signaling pathways that aid in the adaptive immune responses. Similar to other potential adjuvants, LNPs (based on the composition) also induce the activation of several signaling pathways, including nuclear factor kappa B, interferons, inflammasome activation, and other
[5][46].
In the future, it will be essential to compare and understand the immune pathways activated by different mRNA vaccine platforms, improve current approaches based on these mechanisms, and start new clinical trials against more disease targets.
3. Salient Features of LNP-Based Therapeutics
+ T cells) and release several cytokines and chemokines, which further help in the shaping the antigen-specific immune responses, including B cell differentiation and plasma cell development
[13][69]. The two successful vaccines (Pfizer/BioNTech and Moderna’s Spikevax) have confirmed this mechanism in both preclinical and clinical studies (
Figure 26)
[14][70].
Table 12. FDA approved COVID-19 mRNA vaccines
[15][16][71,72].
-
Naked mRNA is unsuitable for therapeutic purposes, as it is rapidly degraded by extracellular RNases. Several nanotechnology platforms have been set and optimized for mRNA-targeted delivery.
-
As mRNA is thermolabile, LNPs would enhance its stability and half-life at room temperature and be useful for avoiding vaccine cold chain.
-
Modifications of the type of delivery system (carrier molecules) rule out the organ-specific mRNA delivery (for lung, spleen, liver, etc.) and its in vivo half-life.
-
Composition of LNP could decide the type of immune response induced.
-
LNPs act as adjuvants systems for mRNA vaccines.
-
The stable LNPs make the lower dose of mRNA work.
-
Composition of LNPs also decides the number of booster doses required if it is admixed with a suitable adjuvant.
-
Moreover, for chronic treatments, multiple administration via different routes of administration is possible.
4. Mechanism of mRNA Vaccines
Even though the needle-free route of administration is long awaited
[6][7][8][62,63,64], mRNA encapsulated by LNPs is administered mostly by the intramuscular route, and several study results support this route of administration
[9][10][11][65,66,67]. Upon injection, local innate immune cells are recruited to the site of injection, followed by the uptake of LNPs by innate immune cells
[12][68]. Once the LNPs reach the cytosol, lower pH enables the LNPs to release mRNA, followed by the translation of targeted antigens (
Figure 15). The antigens are presented to the T helper cells (CD4
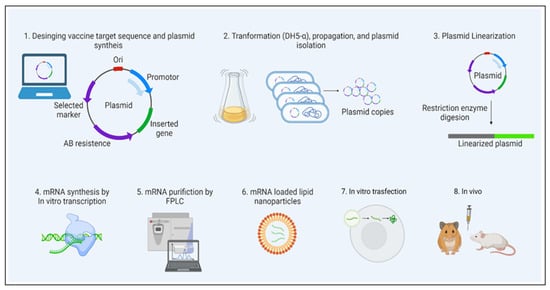
Figure 26. mRNA vaccine development. The figure illustrates the sequential steps involved in the mRNA vaccine development, from design to preclinical studies. Prior to going on to the next step, quality evaluation is required at each step.