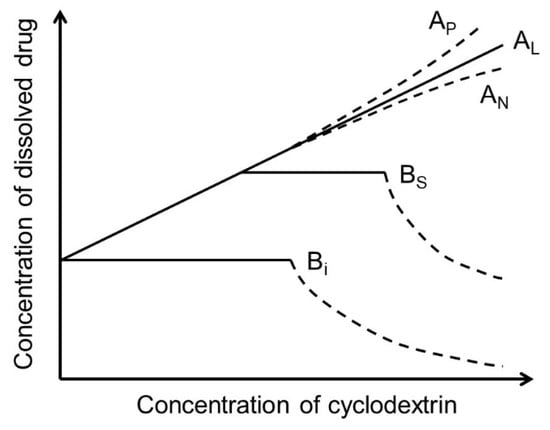
Cyclodextrins (CDs) are cyclic oligosaccharides that emerged as industrial excipients in the early 1970s and are currently found in at least 130 marketed pharmaceutical products, in addition to numerous other consumer products. Although CDs have been the subject of close to 100,000 publications since their discovery, and although their structure and properties appear to be trivial, CDs are constantly surprising investigators by their unique physicochemical properties. In aqueous solutions, CDs are solubilizing complexing agents of poorly soluble drugs while they can also act as organic cosolvents like ethanol. CDs and their complexes self-assemble in aqueous solutions to form both nano- and microparticles. The nanoparticles have diameters that are well below the wavelength of visible light; thus, the solutions appear to be clear. However, the nanoparticles can result in erroneous conclusions and misinterpretations of experimental results. CDs can act as penetration enhancers, increasing drug permeation through lipophilic membranes, but they do so without affecting the membrane barrier. This review is an account of some of the unexpected results the authors have encountered during their studies of CDs as pharmaceutical excipients.
- cyclodextrins
- properties
- aggregation
- nanoparticles
1. Cyclodextrins as Complexing Agents
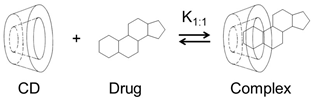
| Cyclodextrin | Abbrev. | Pharmacopoeia Name | Molecular Weight b | Solubility in Water (mg/mL) e | LogP f | H-Acceptors g | H-Donors g |
|---|---|---|---|---|---|---|---|
| α-Cyclodextrin | αCD | Alfadex | 973 | 129.5 | −13 | 30 | 18 |
| β-Cyclodextrin | βCD | Betadex | 1135 | 18.4 | −14 | 35 | 21 |
| 2-Hydroxypropyl-βCD | HPβCD | Hydroxypropylbetadex | 1400 c | >600 | −11 | 39 c | 21 c |
| Sulfobutyl ether βCD sodium salt | SBEβCD | Sulfobutylbetadex sodium | 2163 d | >500 | <−10 | 53 d | 15 d |
| γ-Cyclodextrin | γCD | Gammadex a | 1297 | 249.2 | −17 | 40 | 24 |
2. The Anomalous Properties of Water
Water has some unique or anomalous physiochemical properties [12][13][14][15][16][25,26,27,28,29]. The water molecule consists of a highly electronegative oxygen atom that is covalently bound to two weakly electropositive hydrogen atoms that form a 104.5° angle, giving the molecule a close-to-tetrahedral structure. The electrostatic molecular surface forms a dipole where the oxygen atom is partially negative and the hydrogen atoms are partly positive. In liquid water, the polarity of each water molecule results in an intermolecular attraction and hydrogen bond formations. On average, each water molecule forms about 3.6 hydrogen bonds with surrounding water molecules; however, importantly, the exchange of hydrogen-bond partners via breaking and reforming occurs in a time range between 1 and 5 picoseconds (ps) [13][17][26,30]. Hydrogen bonds are relatively strong (~5–40 kJ/mol) compared to van der Waals interactions (~1–10 kJ/mol) but much weaker than covalent bonds (~200–1000 kJ/mol). Intermolecular hydrogen bonding of water molecules leads to enhanced molecular cohesion that affects the physiochemical properties. For example, the melting and boiling points of hydrogen sulfide (H2S; MW 34 Da) are −85 °C and −60 °C, respectively, compared to 0 °C and 100 °C, respectively, for water (H2O; MW 18 Da). In fact, if water did not possess this extensive molecular adhesion, its boiling point would not be 100 °C but about −90 °C. This extensive hydrogen bonding also affects other physiochemical properties of liquid water such as its dielectric constant (ε 78.5 at 25 °C), density (1.000 g/mL at 3.98 °C), surface tension, and heat of vaporization (40.65 kJ/mol), making them all higher than expected [12][25]. The dielectric constants of organic solvents, such as ethanol (ε 24.3 at 25 °C) and glycerol (ε 42.5 at 25 °C), are much lower than that of water. Due to self-ionization of the water molecule, protons and hydroxide ions diffuse through water at much faster rate than other ions. For example, protons in the form of hydronium ions (H3O+) diffuse about seven times faster through water than Na+, and OH− diffuses about 2.5 times faster than Cl− [12][25]. In solutions, water molecules form intermolecular and directional interactions that give rise to a wide variety of molecular networks that are constantly being formed and disassembled. In aqueous solutions, hydration shells of structured water molecules are formed around solute molecules and at membrane surfaces [13][18][26,31]. In the case of hydrophilic solutes, such as polar molecules (e.g., CDs), the partly positive and partly negative surfaces of the water molecule form hydrogen bonds with the solute molecules. Formation of hydrogen bonds is thermodynamically favored. Nonpolar solutes, like many Class II drugs of the biopharmaceutics classification system (BCS), are hydrophobic and unable to form hydrogen bonds with water. Instead, water molecules form structured layers of water around the hydrophobic solute molecules that tend to aggregate to minimize the contact area of the hydrophobic surface with water. Hydration of hydrophobic solutes involves disruption hydrogen bonds and loss of entropy; thus, it is not thermodynamically favored, which can explain their low aqueous solubility. The structured water layers at membrane surfaces can hamper the permeation of drug molecules through membrane surfaces and decrease the rate of drug permeation through biomembranes such as mucosa [19][20][21][22][32,33,34,35]. Stable CD hydrates contain several water molecules. For example, in the solid state 2, 6, and 8.8 water molecules are present in the αCD, βCD, and γCD cavities, respectively, and 4.4, 3.6, and 5.4 water molecules, respectively, are spread around the CD exterior [9][22]. In bulk water, each water molecule forms on the average 3.6 hydrogen bonds, whereas, inside the αCD, βCD, and γCD cavities, each water molecule forms 1.5, 1.9, and 2.2 hydrogen bonds, respectively [23][36]. Thus, expulsion of water from the central CD cavity by a lipophilic drug moiety is thermodynamically favored. In aqueous solution, each glucose repeat unit of a CD molecule forms about 4–5 hydrogen bonds with surrounding water molecules, which create the first hydration shell, in addition to intramolecular hydrogen bonds between C2–OH on one glucose unit and C3–OH of the adjacent glucose unit [23][24][36,37]. It has been proposed that the ability of αCD, βCD, and γCD to form intra- and intermolecular hydrogen bonds can explain their differences in aqueous solubility [1][25][26][14,38,39]. Chemical substitution of OH groups of the CD molecules (e.g., in HPβCD and SBEβCD) has a significant effect on the hydrogen bonding of water molecules in the CD hydration shell [27][40]. Nevertheless, the main reason for the enhanced aqueous solubility of HPβCD and SBEβCD is the average degree of substitution (i.e., number of substituents per cyclodextrin molecule) and the random distribution of the substituents that results in amorphous mixtures of substituted CD isomers. The βCD molecule contains 21 hydroxyl functional groups that allow numerous possible combinations for substitution. Furthermore, the 2-hydroxypropyl moiety contains an additional optical center and, thus, the number of geometrical and optical isomers of HPβCD can be astronomical [28][41]. Studies have shown that both the molar substitution and the location of the substituents can influence the aqueous solubility and complexing behavior of HPβCD, as well as of other substituted CDs [29][42].3. The Anomalous Properties of Cyclodextrins
CDs are small cyclic glucose polymers, or oligosaccharides, with molecular weight between 973 and 1297 Da. In spite of their rather trivial structure, their physiochemical properties have been shown to be far from predictable. In fact, these rather simple molecules are constantly surprising pharmaceutical formulators with their unexpected properties, especially in aqueous solutions, most of which can be explained by their inter- and intramolecular hydrogen bonding, as well as by the anomalous properties of water. It should also be mentioned that, while theoretical chemistry is frequently based on studies in close-to-ideal solutions (i.e., very dilute solutions), pharmaceutical solutions are nonideal solutions (i.e., concentrated solutions of drugs and excipients) and, thus, can deviate from the classical physicochemical principles.
