Pterostilbene is a natural 3,5-dimethoxy analog of resveratrol. This stilbene compound has a strong bioactivity and exists widely in Dalbergia and Vaccinium spp. Besides natural extraction, pterostilbene can be obtained by biosynthesis. Pterostilbene has become popular because of its remarkable pharmacological activities, such as anti-tumor, anti-oxidation, anti-inflammation, and neuroprotection. Pterostilbene can be rapidly absorbed and is widely distributed in tissues, but it does not seriously accumulate in the body. Pterostilbene can easily pass through the blood-brain barrier because of its low molecular weight and good liposolubility. In this review, the studies performed in the last three years on resources, synthesis, bioactivity, and pharmacokinetics of pterostilbene are summarized.
- pterostilbene
- synthesis
- bioactivity
- pharmacokinetics
- research progress
1. Introduction
Pterostilbene (3,5-dimethoxy-4′-hydroxy-trans-stilbene) is a trans stilbene compound with bioactivity. It was extracted and isolated from the heartwood of Pterocarpus marsupium for the first time in 1940 [1]. The existence of polyphenols, such as resveratrol and pterostilbene, in Darakchaava, a famous Indian herbal medicine, was studied by high performance liquid chromatography. These phenolic compounds are known antioxidants and are used in cancer chemoprophylaxis. They can reduce mortality from coronary heart disease by increasing high-density lipoprotein content and inhibiting platelet aggregation [2]. Considering these findings, resveratrol and pterostilbene have become the focus of research.
Extract from the genus Dracaena (Dragon’s blood) is a renowned traditional medicine in various cultures around the world [3]. Dragon’s blood extract has traditionally been used as a wound healing and antineoplastic agent because of its antibacterial, antioxidant, anti-inflammatory, and anti-apoptotic activities [4][5][6][4–6]. Resveratrol is a popular molecule that has been widely studied in recent years, and it is one of the main components of the extract of Dragon’s blood. Resveratrol and pterostilbene have similar structures, and pterostilbene is also a main bioactive compound in the extract of Dragon’s blood. Pterostilbene shows anti-inflammatory [7], anti-oxidant [8], anti-tumor [9], neuroprotective [10], lipid-lowering [11], and hypoglycemic [12] activities. Similar to other stilbene compounds, pterostilbene has two more methoxy groups on the A-benzene ring than resveratrol, indicating its higher liposolubility, which may lead to the increased permeability of the cell membrane. In many cases [13][14][15][11,13–15], pterostilbene showed significantly higher bioactivity than resveratrol.
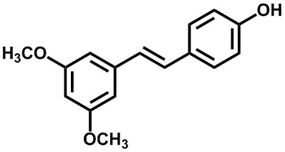
An outstanding drug should have excellent bioactivities and appropriate pharmacokinetic parameters. Pterostilbene is characterized by low molecular weight (Figure 1) and good liposolubility, allowing it to easily cross the blood-brain barrier [16]. Experiments showed that pterostilbene can be rapidly absorbed and is widely distributed in the body. It has suitable metabolic stability and bioavailability [17][18][17,18], indicating its suitable pharmacokinetic characteristics. In addition, most available data in human and animal models show that pterostilbene has no significant toxic effects [19][20][19,20]. Therefore, pterostilbene is a potential natural small-molecular medicine with a good development prospect. This article reviews the progress in pterostilbene synthesis, bioactivities, and partial mechanisms and certain drug metabolism characteristics in vitro and in vivo. This review provides advantageous information for the comprehensive study of the pharmacokinetic features and the mechanisms underlying the bioactivity of pterostilbene.
Figure 1. Chemical structure of pterostilbene.
2. Resources and Synthesis
2.1. Resources
Pterostilbene has a wide range of natural sources, such as Dalbergia and Vaccinium spp. Devaiah et al. collected berries in 2013, 2014, and 2015 from 42 grape varieties and assessed the ability of each grape variety to continuously produce four major stilbene compounds, namely, t-piceid, t-resveratrol, ε-viniferins, and t-pterostilbene. Pterostilbene widely existed in the different grape varieties [21]. Rimando et al. found pterostilbene in two Vaccinium (V. ashei) varieties, and its content in the dried sample was 99–520 ng/g [22].
2.2. Chemical Synthesis and Biosynthesis of Pterostilbene
In addition to being extracted from natural products, pterostilbene can also be synthesized by biological and chemical methods. The chemical synthesis of pterostilbene involves the following methods.
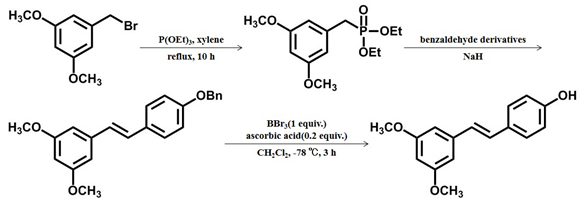
- Pterostilbene was synthesized from 3,5-disubstituted benzyl bromide by Arbuzov, Wittig–Horner reaction and deprotection. Among them, boron tribromide and ascorbic acid were used in the process of deprotection, and the use of ascorbic acid can reduce the occurrence of polymerization in the process of deprotection [23]. This method has some shortcomings, such as purification and separation difficulties, high environmental pollution, and low synthesis yield.
Scheme 1 The first chemical synthesis method of pterostilbene[23] [23].
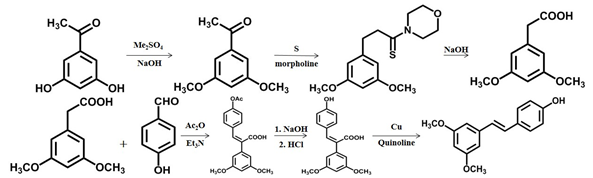
- Pterostilbene was obtained from 3,5-dihydroxyacetophenone by methylation, rearrangement, perkin reaction, and decarboxylation-isomerization reaction [24]. Impurities were formed, and the yield was low due to the high decarboxylation temperature.
Scheme 2 The second chemical synthesis method of pterostilbene [24].
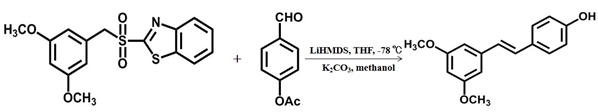
- Pterostilbene was synthesized through the Julia olefin reaction of 3,5-dimethoxybenzyl sulfone derivative and 4-acetoxy benzaldehyde under the action of hexamethyldisilylamino lithium [25], its further industrial application was limited by low temperature conditions.
Scheme 3 The third chemical synthesis method of pterostilbene [25].
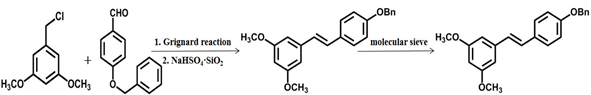
- The trans stilbene skeleton was prepared by Grignard reaction and solid acid catalytic dehydration. The yield is approximately 77%, but controlling the anhydrous condition of grignard reagent in industrial scale-up production is an urgent problem [26].
Scheme 4 The fourth chemical synthesis method of pterostilbene [26].
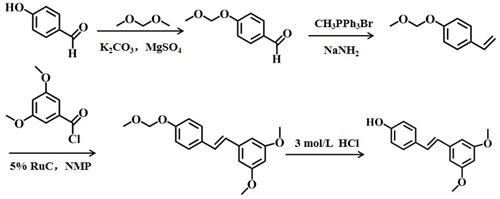
Shi et al. [27] studied a new process (Scheme 5). The yield of the process was approximately 78%, the reaction conditions were mild, the market supply of raw materials was sufficient, and no special low temperature or pressure device was used. The whole process did not need column separation or distillation, and the product was purified only by recrystallization, which was suitable for industrial production.
Scheme 5 The fifth chemical synthesis method of pterostilbene [27].[27]
Biosynthesis plays an important role in the synthesis of pterostilbene because of its high yield and low production cost. Resveratrol O-methyltransferase and grapevine stilbene synthetase can catalyze the resveratrol biosynthesis of pterostilbene in tobacco by agricultural osmosis technique; resveratrol O-methyltransferase gene expression in grape leaves was induced by different stress treatments [28]. First, Heo et al. first used an engineered strain to increase l-tyrosine, the initial precursor of pterostilbene. Second, they tried to use a medium containing l-tyrosine to produce pterostilbene in engineered Escherichia coli and obtained up to 33.6 ± 4.1 mg/L of pterostilbene in a minimal medium containing 1 mM l-methionine, which was about 3.6 times higher compared with that obtained from the parental E. coli strain harboring a plasmid for pterostilbene biosynthesis [29][30][29,30]. In the experiment of Kallscheuer et al., the synthesis of MalEEc-OMTVv fusion protein enabled the constructed Corynebacterium glutamicum strain to produce pterostilbene with a titer of 42 mg/L (0.16 mM) after 6 days of culture, thereby proving that C. glutamicum was suitable for the microbial production of pterostilbene [31].
3. Pharmacokinetics
Pterostilbene has suitable pharmacokinetic characteristics and no significant toxic effects. Although many biological similarities exist between pterostilbene and resveratrol, pterostilbene shows better bioactivity and bioavailability.
3.1. Absorption
Pterostilbene has more pharmacokinetic advantages than resveratrol. For instance, it can be rapidly absorbed. A pharmacokinetic study showed that at a single oral dose (pterostilbene 56 mg/kg, resveratrol 50 mg/kg), the peak plasma concentration (Cmax) values of pterostilbene were 36 times higher than those of resveratrol, the time to maximum plasma concentration (Tmax) value of pterostilbene was twice as fast as resveratrol The oral bioavailability of pterostilbene was 66.9%, whereas that of resveratrol was 29.8% [17]. The area under the curve (AUC) values of pterostilbene in Caco-2, HT29, and HCT116 cells were 2.6, 4.1, and 2.2 times higher than those of resveratrol, respectively [32][33][14,32,33]. From the perspective of chemical structure, pterostilbene has good bioavailability, probably owing to the presence of two additional methoxy groups on the A-benzene ring of resveratrol. These two methoxyl groups are responsible for its high lipophilicity, which may increase the permeability of the cell membrane and pterostilbene’s oral absorption. Experiments using Caco-2 cells as an in vitro model of human intestinal absorption showed that the absorption of pterostilbene prodrug containing isoleucine is due to passive diffusion and the expressions of H+-dependent transporters, such as PepT1 and OATP, on the apical membrane of intestinal cells [34]. Pterostilbene can be absorbed into the systemic circulation through the oral mucosa, thus, mixing pterostilbene into chewing gum or lozenges can be an innovative strategy to prevent oral cancer [34].
One of the main factors affecting the absorption of pterostilbene is the poor solubility (about 21 μg/mL). Different methods have been used to improve the water solubility of the compound. (1) When pterostilbene was co-crystallized with piperazine at a stoichiometric molar ratio of 2:1, its water solubility increased six-fold [1]. (2) The bioavailability of pterostilbene also improved by solubilized excipients such as 2-hydroxypropyl-β-cyclodextrin (HP-β-CD). The bioavailability of HP-β-CD pterostilbene solution (F = 59.2 ± 19.6%) was better than that of pterostilbene suspension (F = 15.9 ± 7.8%). The use of pterostilbene–cyclodextrin complex can slow down the rapid metabolism and elimination of pterostilbene, thereby improving its bioavailability [35]. (3) Fasting also affects the absorption of pterostilbene. Food accelerates bile secretion, which increases the water solubility of common compounds. Thus, co-administration of pterostilbene before or after meals can significantly maximize its oral absorption. Under the same dosing route and dose, the bioavailability of the free eating group was three times higher than that of the fasting group. Therefore, the differences in oral pharmacokinetics between suspension and solution and between fasting and non-fasting states were due to the difference in solubility [35]. (4) The modification of pterostilbene into a prodrug containing hydrophobic side chain amino acids can greatly promote its absorption. The hydroxyl group of pterostilbene was reversibly connected to the N-terminal carbamate of natural amino acids, which were easily absorbed by rats after intragastric administration. When using isoleucine or balanine prodrugs, the obtained AUC values of pterostilbene in the blood were highest. Compared with pterostilbene, the administration of isoleucine prodrug increased absorption, decreased metabolism, and maintained high concentrations for several hours in most of the examined organs [34]. The improvement of water solubility promotes the research development of pterostilbene.
3.2. Distribution
Pterostilbene had low molecular weight and good liposolubility, and its distribution volume (5.3 L/kg) was larger than that of whole body water (~0.7 L/kg). Li et al. specifically revealed the tissue distribution of pterostilbene in C57 BL/6 mice [16]. The tissue concentrations at 20 min after oral administration of 28 mg/kg pterostilbene were in the following order: stomach > liver > testis > kidney > intestine > lung > brain > spleen > skeletal muscle > heart. For most tissues, the content of pterostilbene decreased continuously with progressing sampling point (20–90 min), but the highest content of pterostilbene in the brain was 10.3 ± 3.2 μg/g at 45 min, indicating that pterostilbene may easily pass through the blood-brain barrier. Pterostilbene easily crosses the blood-brain barrier because of its good liposolubility. Therefore, in lipid-rich brain tissue, pterostilbene has a higher blood-brain partition coefficient than its sulfated metabolites. The pterostilbene content of some tissues was much higher than that of in the blood [36], which elucidates why pterostilbene can be bioactive even at low blood or plasma concentrations. The above mentioned research shows that pterostilbene is widely distributed in various tissues and may be highly distributed in some organs.
3.3. Metabolism
Phase II metabolism was the main metabolic type of pterostilbene. In male Wistar rats, sulfate was the main metabolite, and only a small amount of glucuronic acid was found in the liver [36]
[37]. As determined by human liver microsomes under the same conditions, 68% of resveratrol was bound to glucuronic acid, more than 75% of pterostilbene remained unchanged, and only 4′-OH could be used for sulfation [38]. Therefore, pterostilbene has high metabolic stability and bioavailability in the human body. However, due to the significant differences in species, dose, and other factors, the metabolic process may also significantly differ.[36]. In mice, glucuronide and sulfate metabolites were the main metabolites of pterostilbene
[37]. As determined by human liver microsomes under the same conditions, 68% of resveratrol was bound to glucuronic acid, more than 75% of pterostilbene remained unchanged, and only 4′-OH could be used for sulfation [38]. Therefore, pterostilbene has high metabolic stability and bioavailability in the human body. However, due to the significant differences in species, dose, and other factors, the metabolic process may also significantly differ.
Intestinal microorganisms may demethylate pterostilbene in CD-1 mouse. However, further studies are needed to confirm this hypothesis [39]. Some studies have shown that pterostilbene significantly inhibits the activities of CYP2C8, UGT1A9, and UGT1A6 in vitro and may inhibit metabolism through these enzymes in vivo [40][41][40,41]. Human UGT1A1 and 1A3 were identified as the two major UGTs responsible for the glucuronic acid oxidation of pterostilbene [39][34,39]. Moreover, Dellinger et al. found the possible existence of gender differences in the metabolism of pterostilbene, which may be due to the difference in the expression of UGT1A1 between sexes [38]. Clinical studies are necessary to evaluate the correlation of these interactions in vivo.
3.4. Excretion
Pterostilbene is excreted in the form of glucuronic acid in male Sprague-Dawley rats. Most of the glucuronic acid-binding metabolites were excreted 12 h after administration, whereas the amount of pterostilbene excreted in urine increased steadily at 120 h after administration. Glucuronic acid-bound pterostilbene metabolites increased at 2 h, indicating their hepatointestinal circulation. These metabolites were eliminated through non-renal pathways, and renal excretion and hepatic excretion accounted for about 0.219% and 99.78% of the total excretion, respectively [42].
At high doses, pterostilbene showed limited elimination ability, because the binding enzyme may be saturated or partially saturated. Pterostilbene showed a lower clearance rate than resveratrol and therefore showed longer therapeutic time window according to Yeo et al. [1] This finding can well prove the structural difference between the two compounds; pterostilbene was less sensitive to binding metabolism. Samuel et al. [35] studied the differences in the clearance rate of pterostilbene at different doses. The clearance rate was 68.2 ± 9.8 mL/min/kg at 2.5 mg/kg and 36.4 ± 7.8 mL/min/kg at 25 mg/kg (Table 1). The clearance rate decreased nearly twice, and the increase of dose led to the decrease of scavenging rate, which may be due to the metabolic saturation or partial saturation of pterostilbene. The limited capacity for pterostilbene elimination prompts researchers to choose doses cautiously in future studies, because even moderate changes in drug metabolic capacity between patients or subjects can lead to significant differences in systemic drug exposure and treatment responses
Table 1. Pharmacokinetics profiles of pterostilbene.
|
Subject |
Mode of Administration |
Dose (mg/kg) |
AUC (mg h/L) |
V (L/kg) |
CL (mL/min/kg) |
T1/2 (min) |
References |
|
C57 BL/6 mice |
iv. |
10 |
26.7 ± 8.2 |
Vc 0.674 ± 0.12 |
(0.014 ± 0.003) × 103 |
34.5 ± 1.0 |
[16] |
|
C57 BL/6 mice |
ig. |
14 |
4.43 ± 2.0 |
Vc 4.9 ± 1.9 |
(0.027 ± 0.008) × 103 |
102± 19.2 |
[16] |
|
C57 BL/6 mice |
ig. |
28 |
11.9 ± 2.1 |
Vc 3.76 ± 0.85 |
(0.012 ± 0.008) × 103 |
87.8 ± 29.2 |
[16] |
|
C57 BL/6 mice |
ig. |
56 |
39.4 ± 9.7 |
Vc 1.57 ± 0.27 |
(0.030 ± 0.005) × 103 |
56.9 ± 13.9 |
[16] |
|
SD rat |
iv. |
2.5 |
(35.6 ± 5.1)/60 |
Vc 2.85 ± 0.50 |
68.2 ± 9.8 |
93.9 ± 22.3 |
[35] |
|
SD rat |
iv. |
5 |
(135,650 ± 8944)/(60 × 103) |
Vc (1267 ± 364)/103 |
37.0 ± 2.5 |
96.6 ± 23.7 |
[43] |
|
SD rat |
iv. |
10 |
(168.7 ± 28.6)60 |
Vc 3.03 ± 0.88 |
59.1 ± 8.8 |
155.1 ± 64.1 |
[35] |
|
SD rat |
iv. |
11.2 |
4009/103 |
Vss 5.30 |
2.7 × 103/60 |
2.9 × 60 |
[17] |
|
SD rat |
iv. |
20 |
17.5 ± 6.6 |
Vd 2.41 ± 1.13 |
(0.960 ± 0.025) × 103/60 |
(1.73 ± 0.87) × 60 |
[39] |
|
SD rat |
iv. |
25 |
(689.2 ± 124.1)/60 |
Vc 2.19 ± 0.17 |
36.4 ± 7.8 |
150.8 ± 15.9 |
[35] |
|
Wistar rat |
iv. |
22.5 |
(38.8 ± 5.3) × 256.3/103 |
Vss 6.1 ± 1.0 |
(2.3 ± 0.3) × 103/60 |
(1.8 ± 0.3) × 60 |
[36] |
CL, clearance; iv., intravenous; ig., intragastric; T1/2, half-life; Vd, volume of distribution; Vss, apparent distribution volume at steady concentration; Vc, apparent distribution volume of central compartment.