Psychedelic-assisted psychotherapy (PAP) has emerged as an alternative strategy, one that arose in response to the crisis of new psychiatric drugs, there have been promising clinical trials of PAP with LSD, psilocybin and ibogaine to combat drug, alcohol and nicotine addiction. Potential applications have been found in the treatment of anxiety, obsessive compulsive disorders, major depression, autism spectrum disorders, and, finally, in delaying cognitive decline.
- psychedelics
- ibogaine
- mescaline
- N,N-dimethyltryptamine
- psilocybin
- psilocin
1. Introduction
2. Tabernanthe Iboga
2.1. Ethnobotany
Tabernanthe iboga is a shrub from equatorial Africa belonging to the Apocinaceae family (Figure 21A). The root is rich in monomeric polycyclic indole alkaloids (1–3% in the whole root, 5–6% in the bark), the primary one being ibogaine, followed by tabernantin, ibogamine, and ibogalin (Figure 21B–E) [35][20]. The root was used by the African natives as a nervous stimulant (to deal with hunger and fatigue) [8]. In addition, it was also used as an aphrodisiac, due to the transient excitement it produces [36][21]. These properties are confirmed in the current uses of the plant as a neuro-muscular tonic, appetite stimulant, anti-asthenic, and antidepressant [37][22]. The psychedelic and hallucinatory action is mainly a result of the ibogaine [35][20]. This alkaloid causes visual hallucinations, often associated with a strong state of anxiety and apprehension [38][23]. At toxic doses, convulsions, bradycardia, hypotension, paralysis, and respiratory arrest occur [38][23].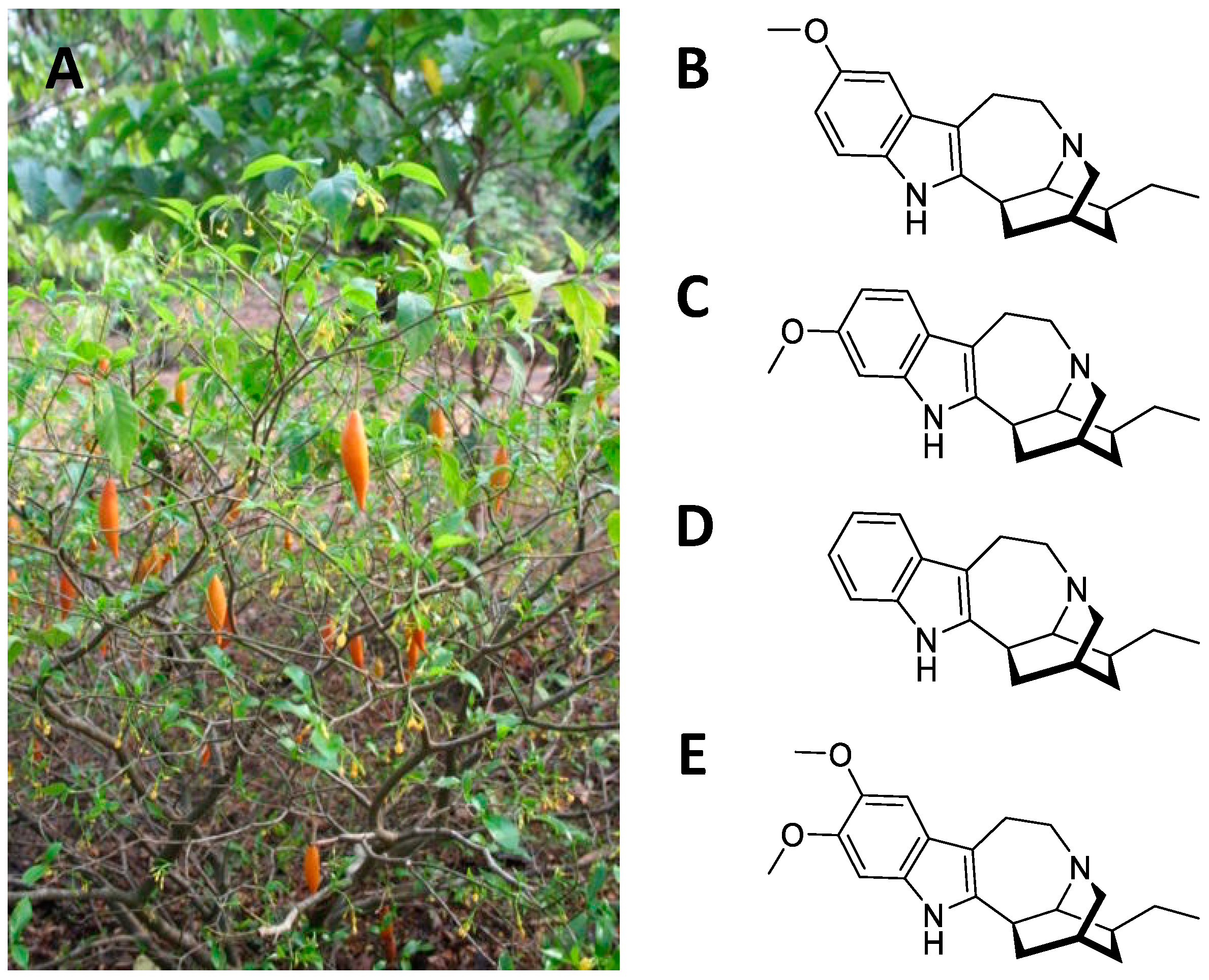
2.2. Central Nervous System Pathways
Early research examined the effects of ibogaine on the central nervous system and on the cardiovascular system [39][24]. Lambarene® was an ibogaine-based drug marketed in France as a “neurostimulant” [39][24]. However, at the end of the 1960s the drug was withdrawn and ibogaine was included among the doping substances in sports and was made illegal in almost all countries [40][25]. Despite the prohibition imposed, results of numerous pharmacological research has suggested potential applications in the treatment of psychological trauma, depressive phenomena, and especially in combating drug addiction [28,36,37,39][21][22][24][26]. Ibogaine is particularly effective when detoxifying from opiates. At the same time, ibogaine exhibits a certain cardiotoxicity [41][27] which has led pharmaceutical chemists to develop new derivatives that act on the opiate pathway and are safe for the cardiovascular system [40][25]. The molecular and receptor mechanisms involved in the action of ibogaine on addictions have yet to be well defined. However, some studies highlight the involvement of neurotrophic factors, monoamine transporters and receptors, and opioid receptors. For example, microinjection of ibogaine in the ventral tegmental area (VTA) induces a reduction of self-administration of ethanol in animal models [42,43][28][29]. This response is blocked after microinjection of anti-GDNF neutralizing antibodies into the VTA [44][30]. In addition, ibogaine has been shown to increase the expression of proBDNF in the nucleus accumbens, a brain area involved in neuronal reinforcement and gratification mechanisms [44][30]. These studies also report that neural growth factor (NGF) is modulated in the mesocorticolimbic area after treatment with ibogaine.2.3. Structural and Computational Studies
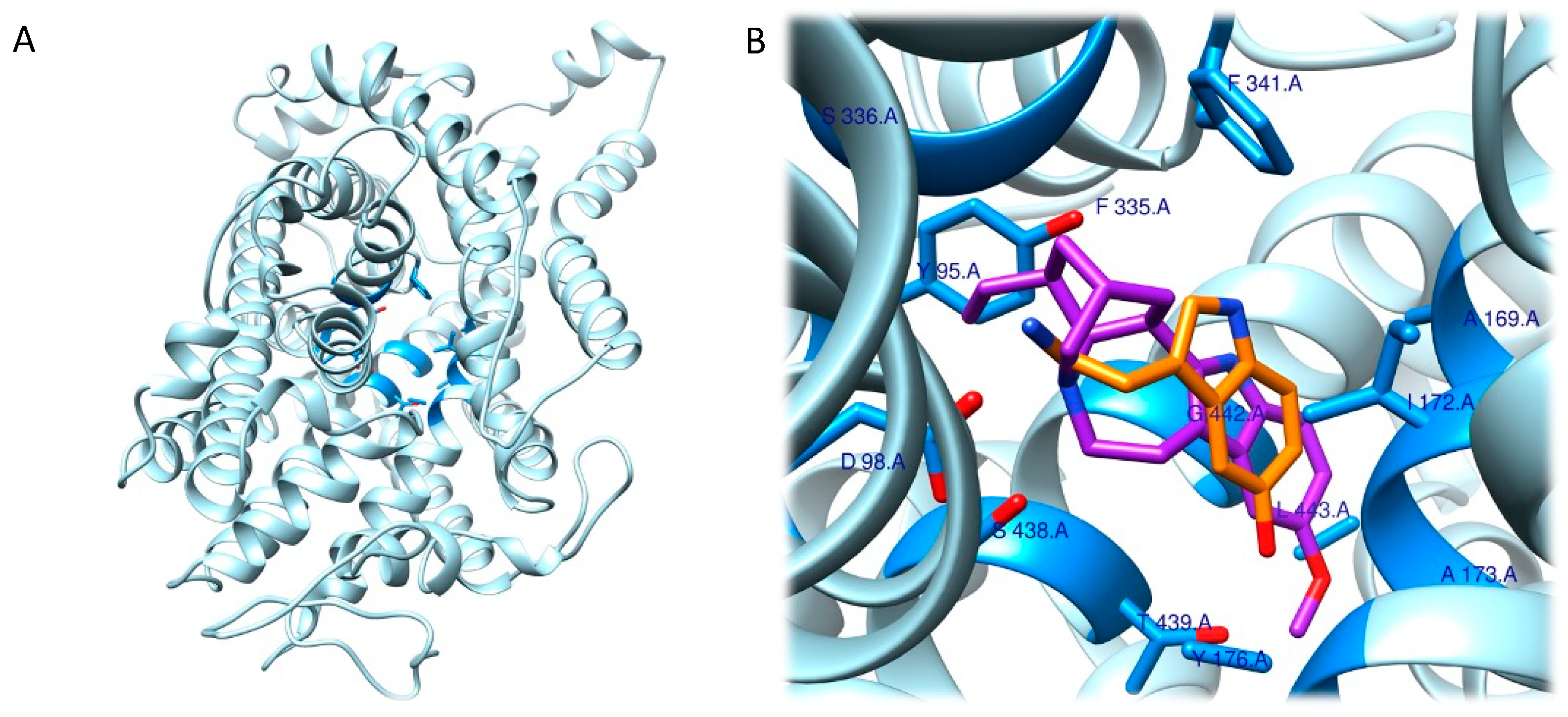
Given the involvement of ibogaine and its metabolites in mood disorders, the molecular docking approach has focused on the human serotonin transporter, hSERT. hSERT is a member of the neurotransmitter sodium symporters (NSSs) family, which is composed of twelve transmembrane secondary active transporters that utilize sodium (Na+) and chloride (Cl−) gradients to promote the transport of neurotransmitter across the membrane in the extracellular fluid [61,62][31][32] while e potassium ion (K+) antiport stimulates the transport process in the cytoplasmic environment [63,64][33][34]. hSERT is a major target of antidepressants and of ibogaine metabolites. The function of NSSs can be modulated by a set of small molecules, which control the availability of neurotransmitter at synapses. hSERT functioning can be summarized with the serotonin reuptake process [63][33]. Given the association between hSERT’s mechanism of action and different conformational states, Coleman et al. were able to resolve the structure of three of these hSERT-ibogaine arrangements, which were deposited in the Protein Data Bank (www.rcsb.org; accessed on 30 November 2022) and are identified by the following codes: 6DZY (outward-open), 6DZV (occluded), and 6DZZ (inward-open) [57,62,65][32][35][36].
The schloars speculated that, because it was impossible for ibogaine to access the central binding pocket of the occluded hSERT, it might bind to the inward- or outward-open conformation and remain bound there through the allosteric binding site so that it could then access the active site in a second step through conformational changes. Another aspect was then considered: the steric bulk of ibogaine (310.4 g/mol) is higher than that of serotonin (176.2 g/mol), which excludes it from being a competitive serotonin substrate candidate because it cannot fit completely into the central binding site. This further supports the hypothesis that considers ibogaine to be a non-competitive ligand for the central binding site capable of stabilizing the inward- and outward-open conformations of hSERT. The information obtained is crucial for future studies aimed at understanding the complete functioning of small molecules, such as ibogaine, that are capable of selectively binding the pharmacological target hSERT and for designing or optimizing ligands [57][35] (Figure 2).
2.4. Therapeutic Hypotheses
Over the recent years, ibogaine has been taken into consideration as a therapeutic agent, able to treat or assist the treatments for drug addictions such as heroin [71][38], cocaine [72][39], methamphetamine [73][40], cannabis and crack [74][41]. The advantage of the use of ibogaine as a therapeutic for drug addiction resides in the fact that a single large dose seems effective in blocking withdrawal and reduces cravings in drug-dependent individuals [72][39], contrary to standard pharmacological treatments which typically require a prolonged administration. Indeed, ibogaine administration, unlike commonly used treatments such as methadone, shortens the time needed for withdrawal to 2–3 days, effectively speeding up the detoxification process [72][39].
Along with the growing use of psychedelics in the treatment of psychiatric disorders, research about the therapeutic effects of ibogaine has begun to take its first steps in this direction. For example, Fernandes-Nascimento et al. (2022) recently reported a single case study of ibogaine microdosing for the treatment of type II bipolar disorder (BPD) [78][42]. Microdosing, a growing trend in the psychedelics field, is defined as recurrent administration of small doses of psychedelic drugs, with little to no identifiable acute drug effects, with the intention of improving mental health and general wellbeing, enhance cognitive performances and boost mood [79,80][43][44]. It reported a significant improvement of symptoms in a 47-year-old woman with a 20-year history of BPD treated for 60 days, with two daily administrations of ibogaine (4 mg per administration, approximately 1% of a full conventional single dose). This finding is consistent with previous preclinical studies undertaken in rats [81][45], which characterized behavioural effects induced by acute ibogaine and noribogaine (20 and 40 mg/kg, i.p., single injections for each dose) in rats, assessing depression-like symptoms using the forced swim test. Both drugs induced a dose- and time-dependent antidepressant effect, without significant changes in locomotor activity.
3. Echinocactus williamsii
3.1. Ethnobotany
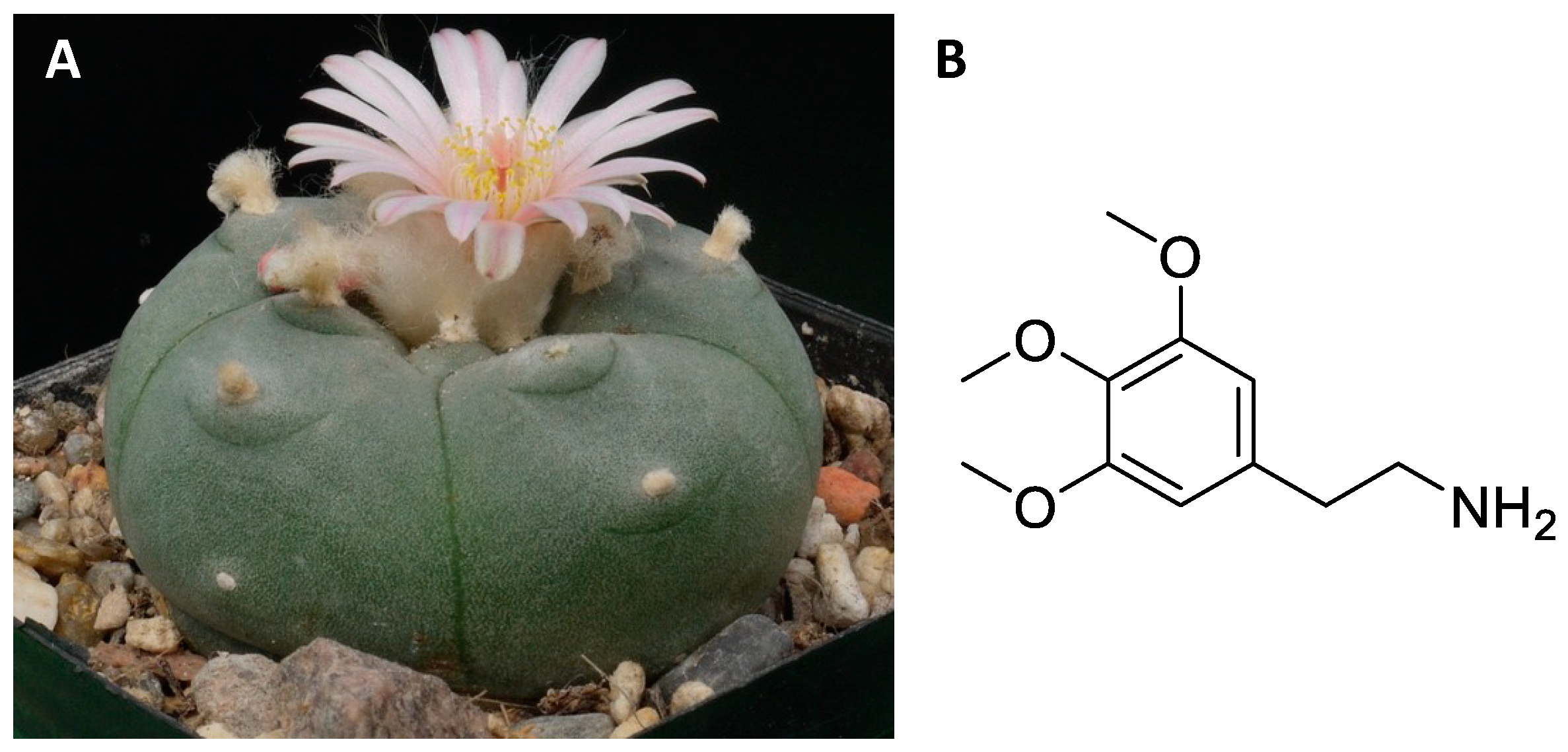
3.2. Central Nervous System Pathways
Regarding the pharmacological aspects, mescaline is considered an agonist of the 5-HT2A and 5-HT2C receptors of serotonin, and of the α2A adrenergic receptor [90,93,94,95][54][57][58][59]. In contrast, mescaline shows low affinity for other serotonergic receptors and for dopamine receptors and monoamine transporters [85,91,93,94,95][49][55][57][58][59]. The hallucinogenic effects appear to be regulated by mescaline agonism towards the 5-HT2A receptor [85,95][49][59]. However, it has been observed in animal models that blockade of dopamine receptors with haloperidol can halt the characteristic effects of mescaline, suggesting the additional involvement of other neuronal pathways [91][55].
3.3. Structural and Computational Studies
3.4. Therapeutic Hypotheses
Mescaline, along with other psychedelics such as LSD and psilocybin, has been widely employed in psychiatric and therapeutic contexts, before its use was restricted by the Schedule I of the UN Convention on Drugs in 1967 [91][55]. Due to its regulation, clinical research over recent decades has been heavily limited [90][54]. Preclinical studies using animal models have been employed to investigate the effect of mescaline on behaviour. As mentioned previously, mescaline is able to induce an increase in locomotor activity and exploratory behaviour, although some studies report a dose-dependent reduction with an inverted U-shape curve (locomotion and exploratory behaviour increase at lower doses and decrease at higher doses) [102,103,104][61][62][63]. Mescaline is also able to induce an increase in animal reactivity [105][64].
4. Psychotria Viridis
4.1. Ethnobotany
4.2. Central Nervous System Pathways
4.3. Structural and Computational Studies
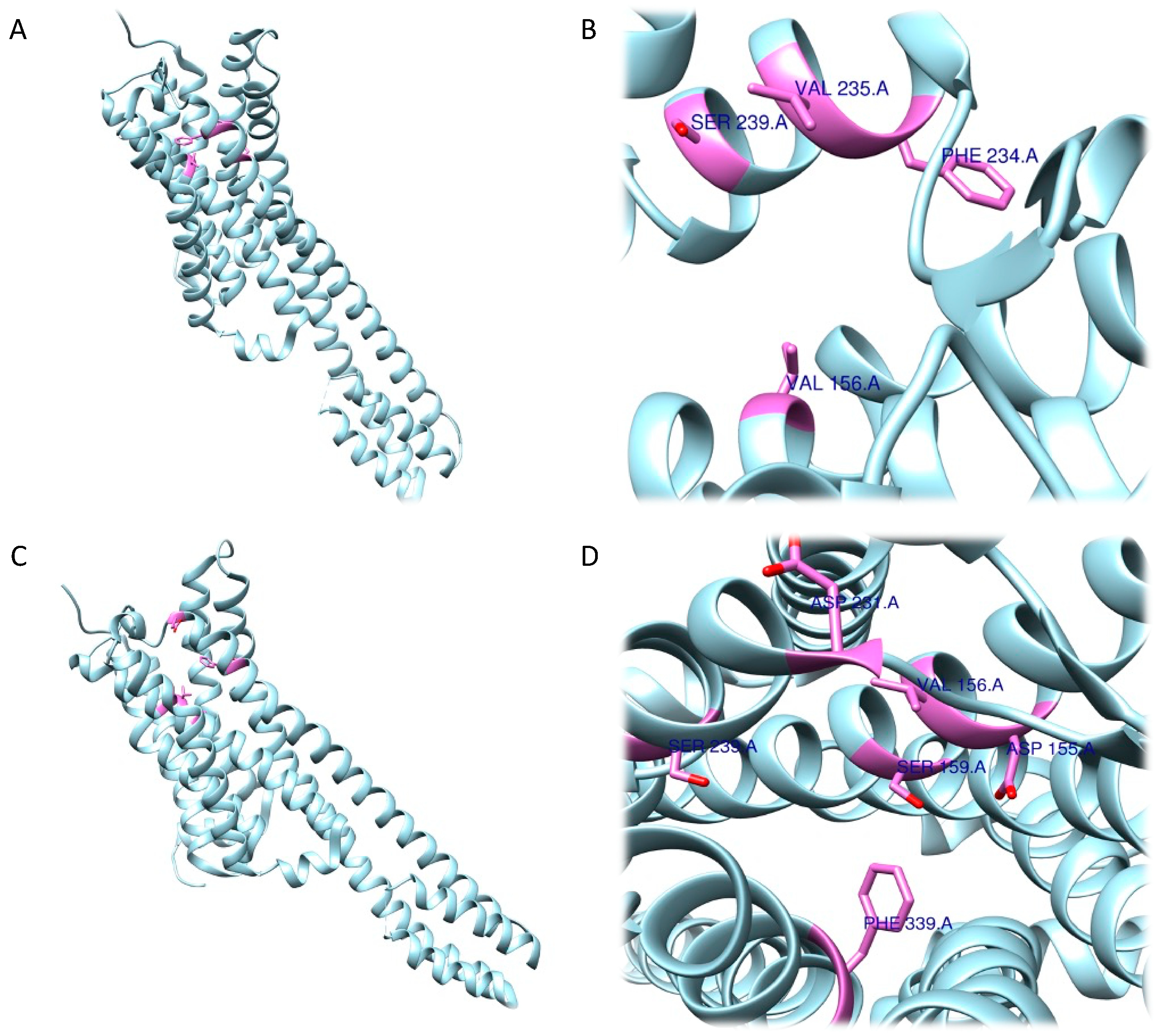
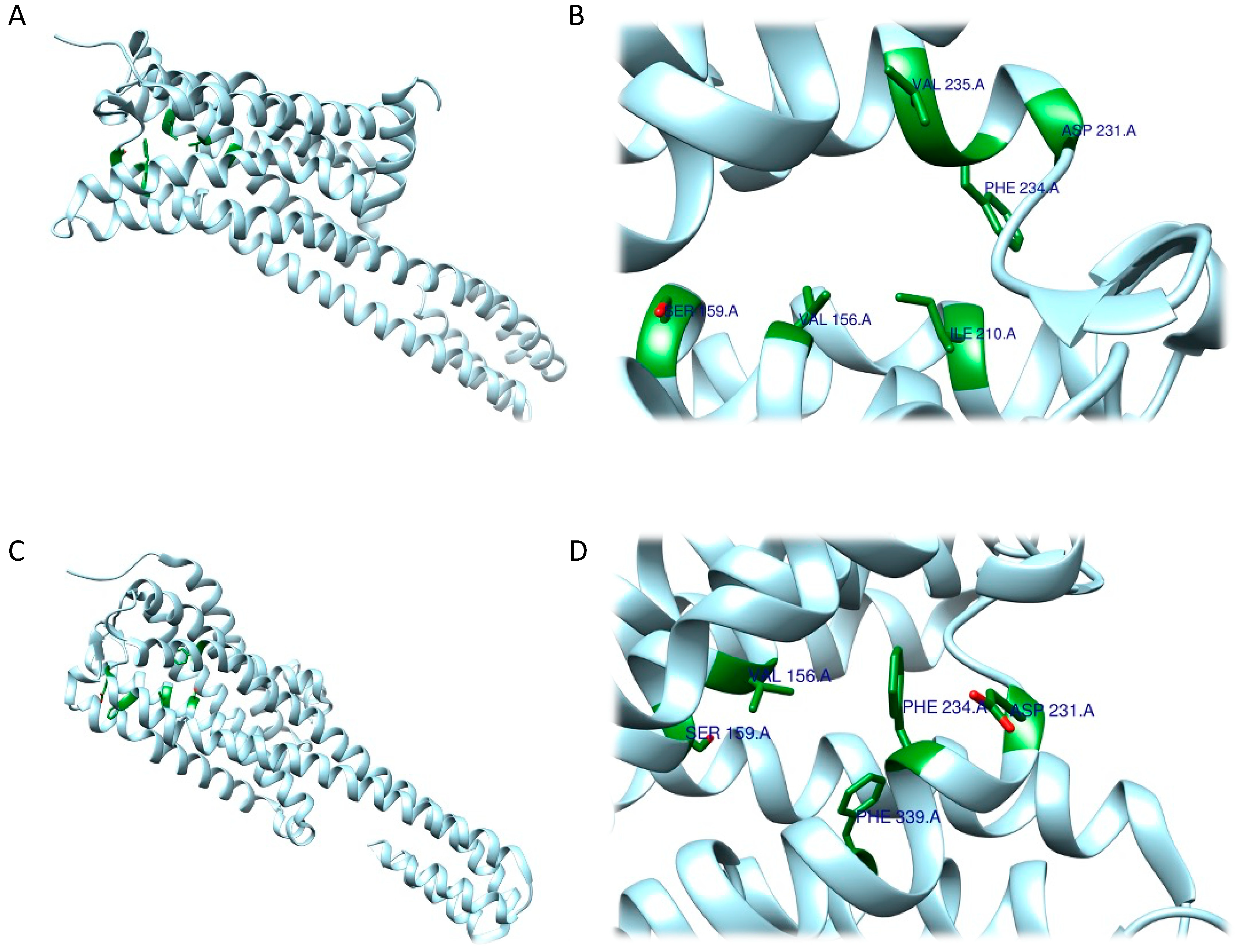
The RRA shows a π–σ interaction with Ile210 (2.83 Å), a H bond between the H(N) atom of the indole moiety and Asp231 (2.17 Å), a H bond between NHMe2 and Ser159 (3.43 Å), an amide–π stacking interaction with Phe234 (3.98 Å), an amide–π stacking interaction with Phe234 (4.12 Å), a π–alkyl interaction with Val235 (4.95 Å) and Val156 (5.01 Å), and a π–alkyl interaction with Ile210 (5.00 Å) and Val156 (4.95 Å). Once the interactions were identified, they were classified according to the distance between the atoms involved as strong (distance ≤ 3 Å), intermediate (distance between 3–5 Å), and weak (distance ≥5 Å). Overall, two strong, five intermediate, and two weak interactions were detected (Figure 96A,B).
4.4. Therapeutic Hypotheses
Although DMT is known for its psychedelic properties, latest research has shown its clinical utility, addressing a variety of medical conditions (especially those of the central nervous system). As a matter of fact, in the last decade DMT has been investigated for its neuroprotective effects, which are thought to be mediated by binding with the sigma-1 receptor [145][89]. In-vitro experiments have displayed DMT’s cytoprotective effect against hypoxia: diverse cell types (human cortical neurons derived from iPSC, dendritic cells, monocyte-derived macrophages) cultured in severe hypoxic conditions have been treated with DMT. Results suggest that DMT is able to prevent cellular stress and robustly boosts cell survival [146][90]. DMT is also able to activate the subgranular zone of the dentate gyrus in-vivo, a part of the hippocampal formation in which most adult neurogenesis takes place. This neurogenic propriety seems to have a robust functional effect, since DMT-treated mice (2 mg/kg, i.p., for 21 days) performed better in memory and learning tasks, for which the hippocampus plays an essential role [148][91]. The increased neurogenesis and subsequent cognitive improvement provided by DMT treatment could have profound implications for neurodegenerative diseases such as Parkinson’s (PD) and Alzheimer’s (AD) and for medical conditions defined by significant loss of neural cells in affected areas of the CNS, such as stroke. Previous literature [149,150,151,152][92][93][94][95] indicates that neurogenesis and neuroprotective factors could be implemented as a valid tool in the therapeutic strategies for psychiatric and neurological disorders, aiding in the restoration and preservation of residual functionality in the cerebral regions affected by the disease. This concept has recently been assessed using DMT-containing Ayahuasca in an in-vitro model of Parkinson’s disease.
Apart from clinical and preclinical studies, ayahuasca’s therapeutic potential seems to be confirmed by several observational studies, conducted on participants taking ayahuasca in naturalistic setting [163,164,165,166][96][97][98][99]. Although the clear limitation of observational studies, it is important to notice that this field of literature is unanimous in confirming the potential healing properties of ayahuasca and DMT, reporting robust improvements in psychopathological symptoms, boost in overall mood and improvements in mental health well-being that are persistent over time after a single dose.
5. Psilocybe Cubensis
5.1. Ethnobotany
5.2. Central Nervous System Pathways
5.3. Structural and Computational Studies
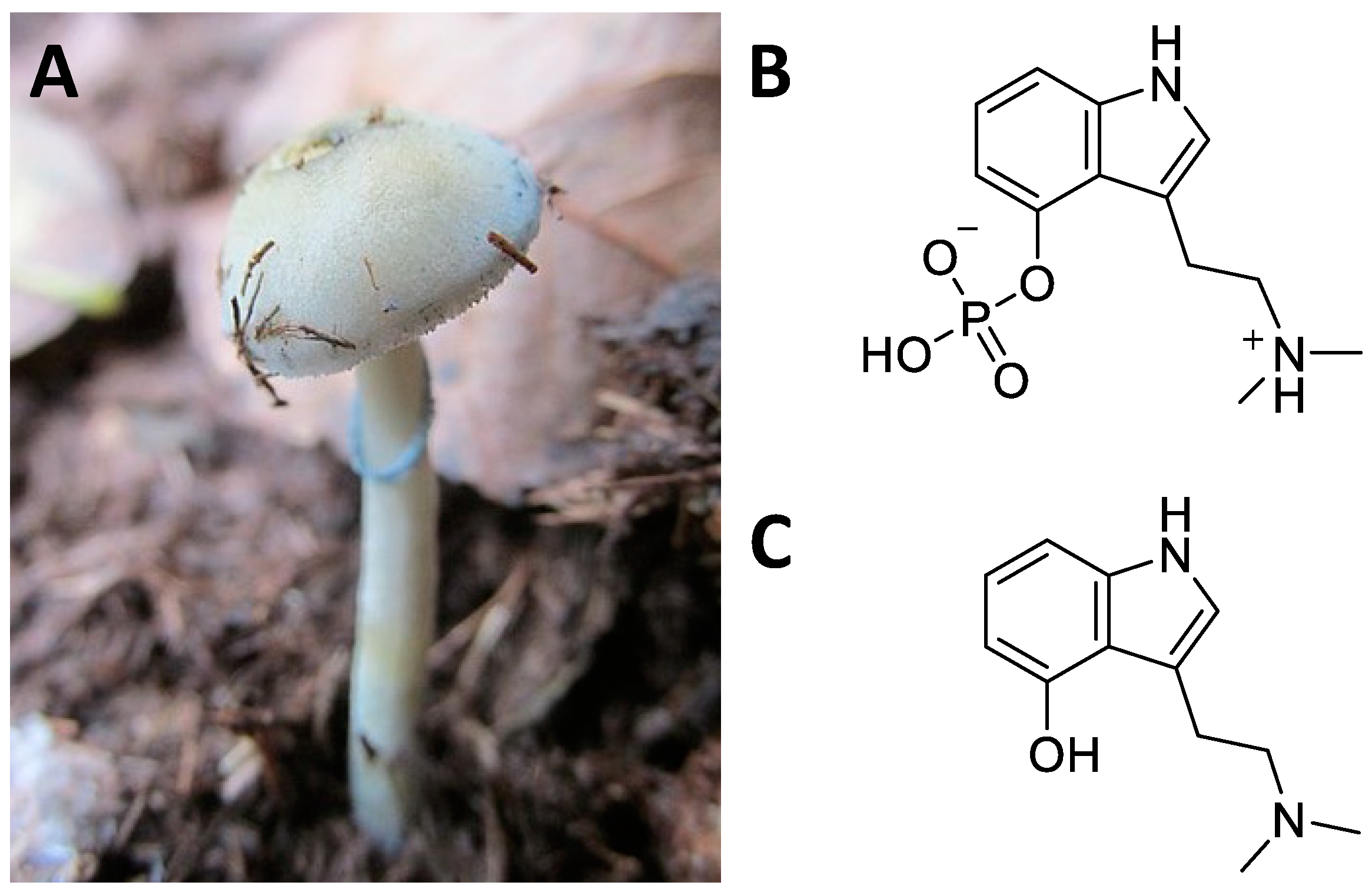
Concerning the 5-HT2A receptor, Cao et al. (2022) resolved and deposited the structure of psilocin-5-HT2A complex (PDB ID: 7WC5) [194,195][114][115]. The work performed by Cao et al. showed that, in addition to the central binding pocket, psilocin is also able to interact with a second binding site where the indole group fits into a pocket, described as an extended binding site, mainly containing hydrophobic residues. A salt bridge is formed between the basic nitrogen and the Asp155 residue and a hydrogen bond between Asn352 and the OH- group in the indole of psilocin was detected. Subsequently, during the analysis of the interactions between the 5-HT2A receptor and psilocin, additional residues involved in the formation of the complex, such as Val156, Phe339, Asn363, Leu228, and Trp151, were identified [195][115].
The study of the role of psilocybin and of its active metabolite then continued, though focused on another receptor, 5-HT2C. In this case, molecular docking was enrolled, and Gumpper et al. (2022) showed that psilocin is also able to bind to the binding pocket of the receptor [196][116]. Here the compound establishes bonds with a salt bridge with Asp134 through the amine compound, a π–stacking interaction between the tryptamine ring and Phe328, and another bond between the indole -OH and Asn331. Further, other interactions with Trp130 and Phe327 were identified in the assembly.
5.4. Therapeutic Hypotheses
6. Claviceps Purpurea
6.1. Ethnobotany
Claviceps purpurea is a fungus, belonging to the Ascomycetes Clavicipetali, which infests cereal crops and in particular rye [209][123] (Figure 158A). The sclerotium, Secale cornutum, is the richest part of the alkaloids (Figure 158B). These include ergolinic alkaloids derived from lysergic acid such as ergotamine, ergometrine, ergocristine, ergocriptine, and ergoconine [210][124]. Alkaloids have found multiple applications in the cardiovascular and gynaecological fields [211][125]. However, the greatest impact on the CNS came with the semi-synthetic synthesis of D-lysergic acid diethylamide (LSD, Figure 158C), deriving from lysergic acid. Indeed, in 1938 the chemist Albert Hofmann synthesized LSD for the first time in the Swiss laboratories of the Sandoz AG Pharmaceutical Company, deriving from lysergic acid, and accidentally tested its hallucinatory and psychedelic effects [212,213][126][127]. LSD has had a major social impact since the 1960s by profoundly influencing Western culture [212,213][126][127]. The cerebral effects of LSD concern the emotional–ideational aspects and above all sensory perception (colours are perceived more vividly) [214,215,216,217][128][129][130][131]. These hallucinogenic effects affect the sight, hearing, touch, and perception of one’s body. Furthermore, subjects who used LSD experienced introspective trips that enabled them to perceive inner problems and reality from other points of view, beyond the usual schemes [214,215,216,217][128][129][130][131].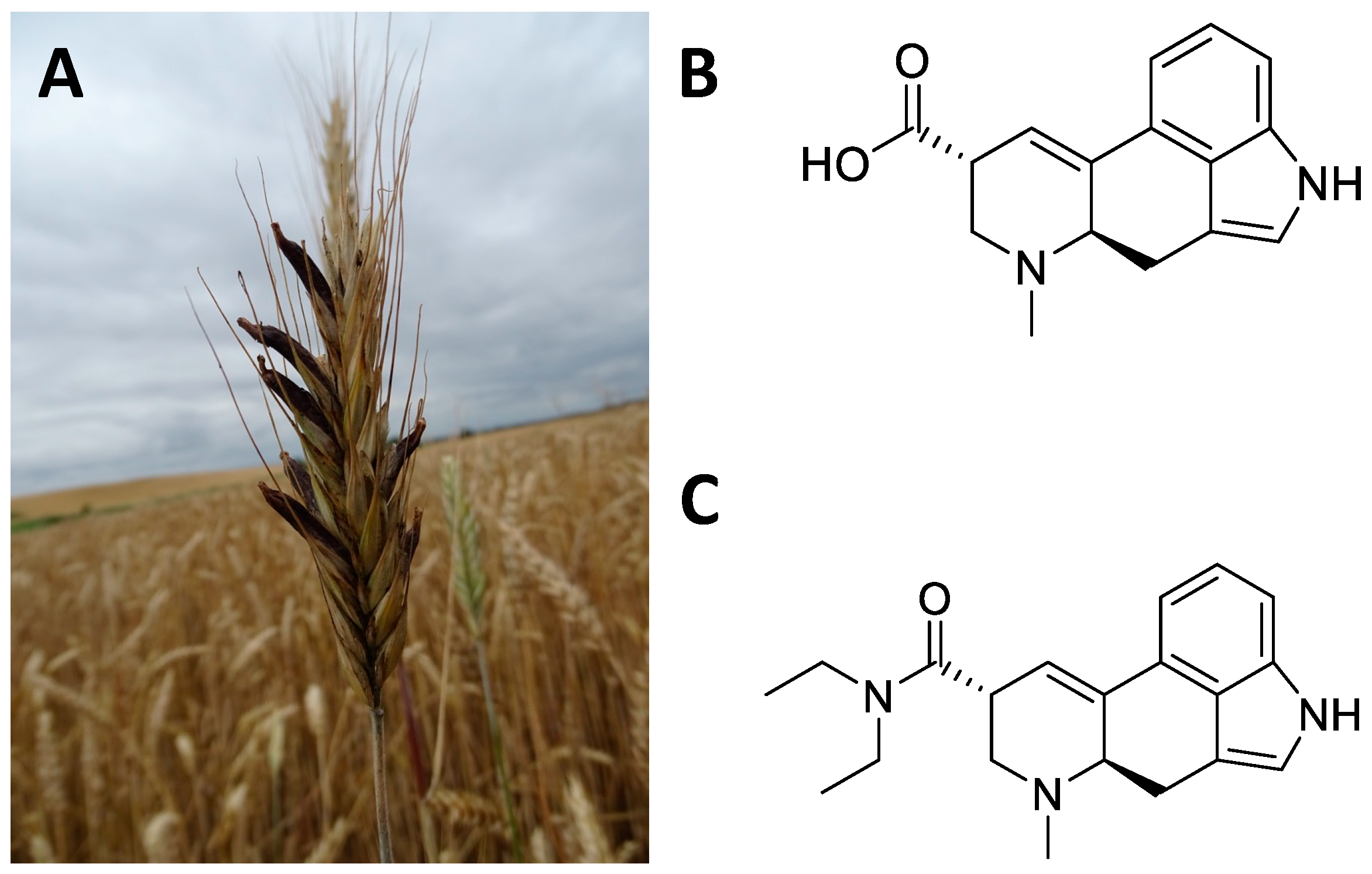
6.2. Central Nervous System Pathways
6.3. Structural and Computational Studies
To assess the action of the LSD, at the level of 5-HT2B receptor, Wacker et al. (2017) characterized the complex through computational studies and structural resolution techniques [234][139]. Their analysis revealed that LSD can bind to the receptor within the orthosteric binding site with a volume of 2898.7 Å3 [235][140]. The ligand has been shown to be able to insert itself into the orthosteric binding pocket through a salt bridge that occurs between Asp135 and the basic nitrogen present in the structure. Subsequently, the schloars identified the way in which the ergoline moiety of LSD can establish aromatic contacts with Phe340 and Phe341, and how the nitrogen in the indole group forms a hydrogen bond with Gly221. Another component capable of forming interactions is the diethylamide moiety, which, thanks to one ethyl group, forms non-polar contacts with Leu132 and Trp131, while the other ethyl group interfaces with Leu362. Further interactions detected are Val136, Ser139, Leu209, Phe217, Ser222, Ala225, Trp337, and Asn344.6.4. Therapeutic Hypotheses
The recent interest in the therapeutic effects of LSD is supported by several preclinical and clinical studies. Concerning preclinical studies, LSD has been tested to treat stress-induced anxiety-like behaviour in mice. In particular, male mice exposed to chronic restrain stress were treated daily with 30 μg/kg LSD for one week and the treatment was able to prevent the stress-induced anxiety-like behaviour, the decrease of cortical spine density and the serotonergic transmission decline [33][141]. Interestingly, LSD treatment did not cause any anxiolytic or anti-depressant effect in non-stressed mice. LSD has also been demonstrated to exert antidepressant effects in rodents and to promote neuroplasticity in rat cortical neurons; a single administration of LSD 0.15 mg/kg i.p. was able to significantly reduce depressive-like behaviours in rats five weeks after treatment. Indeed, by activating the TrkB receptor, mTOR and 5-HT2A signalling pathways in the prefrontal cortex, it produced an increase in dendritic arbor complexity, promote dendritic spine growth, and stimulate synapse formation, thus revealing a fast-acting, robust and persistent antidepressant effect [170,238][142][143]. Additional repeating of LSD administration (0.13 mg/kg) for 11 days was able to reverse deficits in active avoidance learning in bulbectomised rats, a model of depression [214][128]. In healthy human subjects, neuroimaging studies have demonstrated that LSD induces increases in functional brain connectivity between the thalamus and sensory–somatomotor cortical regions, and from the thalamus to the posterior cingulate cortex [216][130]; on the other hand, it decreased connectivity to the temporal cortex [216][130]. These anatomical changes could explain the LSD-induced effect in facilitating a novel experience of the self and its environment and in reducing rigid or ruminative thinking patterns typical of psychiatric disorders [33][141].References
- Lansky, E.S.; Lansky, S.; Paavilainen, H.M. Entheogenesis and Entheogenic Employment of Harmal. Tradit. Herb. Med. Mod. 2017, 20, 55–68.
- Tangarife-Puerta, H.F.; Ceballos-Ceballos, L.A.; Rodriguez-Osorio, J.E. Shamanism, Enthogen and Contemporary Art. Cult. Drog. 2018, 25, 106–136.
- Pothier, B. Potential identification of an entheogenic plant species on the Chi Silk Manuscript. Time Mind 2021, 14, 111–134.
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315.
- Berlowitz, I.; O’Shaughnessy, D.M.; Heinrich, M.; Wolf, U.; Maake, C.; Martin-Soelch, C. Teacher plants—Indigenous Peruvian-Amazonian dietary practices as a method for using psychoactives. J. Ethnopharmacol. 2022, 286, 114910.
- Nemu, D. Getting high with the most high: Entheogens in the Old Testament. J. Psychedelic Stud. 2019, 3, 117–132.
- Carvalhoa, I.C.D.; Steila, C.A.; Gonzaga, F.A. Learning from a more-than-human perspective. Plants as teachers J. Env. Educ 2021, 52, 205.
- Sayin, H.U. The Consumption of Psychoactive Plants During Religious Rituals: The Roots of Common Symbols and Figures in Religions and Myths. Neuroquantology 2014, 12, 276–296.
- Apud, I. Ayahuasca from Peru to Uruguay: Ritual Design and Redesign through a Distributed Cognition Approach. Anthr. Conscious 2015, 26, 1–27.
- Prue, R.; Voss, R.W. Indigenous Healing Practice: Ayahuasca. Opening a Discussion. J. Pastor. Care Couns. 2014, 68, 1–13.
- Crockford, S. Shamanisms and the Authenticity of Religious Experience. Pomegranate 2010, 12, 139–158.
- Ona, G.; Berrada, A.; Bouso, J.C. Communalistic use of psychoactive plants as a bridge between traditional healing practices and Western medicine: A new path for the Global Mental Health movement. Transcult. Psychiatry 2022, 59, 638–651.
- Majic, T.; Sauter, M.; Bermpohl, F.; Schmidt, T.T. Connected to the spirit of the frog: An Internet-based survey on Kambo, the secretion of the Amazonian Giant Maki Frog (Phyllomedusa bicolor): Motivations for use, settings and subjective experiences. J. Psychopharmacol. 2021, 35, 421–436.
- Hartogsohn, I. Modalities of the psychedelic experience: Microclimates of set and setting in hallucinogen research and culture. Transcult. Psychiatry 2022, 59, 579–591.
- Jones, M.T. The creativity of Crumb: Research on the effects of psychedelic drugs on the comic art of Robert Crumb. J. Psychoact. Drugs 2007, 39, 283–291.
- ten Berge, J. Jekyll and Hyde revisited: Paradoxes in the appreciation of drug experiences and their effects on creativity. J. Psychoact. Drugs 2002, 34, 249–262.
- Janiger, O.; Dobkin de Rios, M. LSD and creativity. J. Psychoact. Drugs 1989, 21, 129–134.
- Winkelman, M.J. The Mechanisms of Psychedelic Visionary Experiences: Hypotheses from Evolutionary Psychology. Front. Neurosci. 2017, 11, 539.
- Belouin, S.J.; Henningfield, J.E. Psychedelics: Where we are now, why we got here, what we must do. Neuropharmacology 2018, 142, 7–19.
- Lavaud, C.; Massiot, G. The Iboga Alkaloids. Prog. Chem. Org. Nat. Pract. 2017, 105, 89–136.
- Kohek, M.; Ohren, M.; Hornby, P.; Alcázar-Córcoles, M.Á.; Bouso, J.C. The Ibogaine Experience: A Qualitative Study on the Acute Subjective Effects of Ibogaine. Anthr. Conscious 2020, 31, 91–119.
- Paskulin, R.; Jamnik, P.; Danevcic, T.; Kozelj, G.; Krasovec, R.; Krstic-Milosevic, D.; Blagojevic, D.; Strukelj, B. Metabolic plasticity and the energy economizing effect of ibogaine, the principal alkaloid of Tabernanthe iboga. J. Ethnopharmacol. 2012, 143, 319–324.
- Heink, A.; Katsikas, S.; Lange-Altman, T. Examination of the Phenomenology of the Ibogaine Treatment Experience: Role of Altered States of Consciousness and Psychedelic Experiences. J. Psychoact. Drugs 2017, 49, 201–208.
- Litjens, R.P.W.; Brunt, T.M. How toxic is ibogaine? Clin. Toxicol. 2016, 54, 297–302.
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2020, 589, 474–479.
- Brown, T.K.; Alper, K. Treatment of opioid use disorder with ibogaine: Detoxification and drug use outcomes. Am. J. Drug Alcohol Abus. 2017, 44, 24–36.
- Koenig, X.; Hilber, K. The Anti-Addiction Drug Ibogaine and the Heart: A Delicate Relation. Molecules 2015, 20, 2208–2228.
- He, D.Y.; McGough, N.N.; Ravindranathan, A.; Jeanblanc, J.; Logrip, M.L.; Phamluong, K.; Janak, P.H.; Ron, D. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 619–628.
- Belgers, M.; Leenaars, M.; Homberg, J.R.; Ritskes-Hoitinga, M.; Schellekens, A.F.A.; Hooijmans, C.R. Ibogaine and addiction in the animal model, a systematic review and meta-analysis. Transl. Psychiatry 2016, 6, e826.
- Marton, S.; González, B.; Rodríguez-Bottero, S.; Miquel, E.; Martínez-Palma, L.; Pazos, M.; Prieto, J.P.; Rodríguez, P.; Sames, D.; Seoane, G.; et al. Ibogaine Administration Modifies GDNF and BDNF Expression in Brain Regions Involved in Mesocorticolimbic and Nigral Dopaminergic Circuits. Front. Pharmacol. 2019, 10, 193.
- Torres, G.E.; Gainetdinov, R.R.; Caron, M.G. Plasma membrane monoamine transporters: Structure, regulation and function. Nat. Rev. Neurosci. 2003, 4, 13–25.
- Soares, C.M.; Hellsberg, E.; Ecker, G.F.; Stary-Weinzinger, A.; Forrest, L.R. A structural model of the human serotonin transporter in an outward-occluded state. PLoS ONE 2019, 14, e0217377.
- Jacobs, M.T.; Zhang, Y.-W.; Campbell, S.D.; Rudnick, G. Ibogaine, a Noncompetitive Inhibitor of Serotonin Transport, Acts by Stabilizing the Cytoplasm-facing State of the Transporter. J. Biol. Chem. 2007, 282, 29441–29447.
- Coleman, J.A.; Navratna, V.; Antermite, D.; Yang, D.; Bull, J.A.; Gouaux, E. Chemical and structural investigation of the paroxetine-human serotonin transporter complex. eLife 2020, 9, e56427.
- Coleman, J.A.; Yang, D.; Zhao, Z.; Wen, P.-C.; Yoshioka, C.; Tajkhorshid, E.; Gouaux, E. Serotonin transporter–ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 2019, 569, 141–145.
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980.
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612.
- Mash, D.C.; Kovera, C.A.; Pablo, J.; Tyndale, R.; Ervin, F.R.; Kamlet, J.D.; Hearn, W.L. Ibogaine in the treatment of heroin withdrawal. Alkaloids. Chem. Biol. 2001, 56, 155–171.
- Mash, D.C.; Duque, L.; Page, B.; Allen-Ferdinand, K. Ibogaine Detoxification Transitions Opioid and Cocaine Abusers Between Dependence and Abstinence: Clinical Observations and Treatment Outcomes. Front. Pharmacol. 2018, 9, 529.
- Szumlinski, K.K.; Haskew, R.E.; Balogun, M.Y.; Maisonneuve, I.M.; Glick, S.D. Iboga compounds reverse the behavioural disinhibiting and corticosterone effects of acute methamphetamine: Implications for their antiaddictive properties. Pharmacol. Biochem. Behav. 2001, 69, 485–491.
- Schenberg, E.E.; Comis, M.A.D.; Chaves, B.R.; da Silveira, D.X. Treating drug dependence with the aid of ibogaine: A retrospective study. J. Psychopharmacol. 2014, 28, 993–1000.
- Fernandes-Nascimento, M.H.; Viana-Ferreira, K.; Chaves, B.D.R.; Negrao, A.B.; Wang, Y.P. Ibogaine microdosing in a patient with bipolar depression: A case report. Braz. J. Psychiatry 2022, 44, 462–463.
- Lea, T.; Almada, N.; Jungaberle, H.; Schecke, H.; Klein, M. Microdosing psychedelics: Motivations, subjective effects and harm reduction. Int. J. Drug Policy 2020, 75, 102600.
- Polito, V.; Stevenson, R.J. A systematic study of microdosing psychedelics. PLoS ONE 2019, 14, e0211023.
- Rodriguez, P.; Urbanavicius, J.; Prieto, J.P.; Fabius, S.; Reyes, A.L.; Havel, V.; Sames, D.; Scorza, C.; Carrera, I. A Single Administration of the Atypical Psychedelic Ibogaine or Its Metabolite Noribogaine Induces an Antidepressant-Like Effect in Rats. ACS Chem. Neurosci. 2020, 11, 1661–1672.
- Nuzhyna, N.; Baglay, K.; Golubenko, A.; Lushchak, O. Anatomically distinct representatives of Cactaceae Juss. family have different response to acute heat shock stress. Flora 2018, 242, 137–145.
- Rodriguez, S.M.M.; Rosas, H.G.; de los Santos, G.G.; Garcia-Moya, E.; Espinosa-Hernandez, V.; Torres, T.C.; Robledo-Paz, A. Viability and germination of seeds of four endangered species of cacti. Caldasia 2022, 44, 209–220.
- Hofer, A. (2816) Proposal to conserve the name Echinocactus williamsii Lem. ex Salm-Dyck (Lophophora williamsii) against E. williamsianus Lem. (Cactaceae). Taxon 2021, 70, 676.
- Cassels, B.K.; Saez-Briones, P. Dark Classics in Chemical Neuroscience: Mescaline. ACS Chem. Neurosci. 2018, 9, 2448–2458.
- Carod-Artal, F.J. Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurologia 2015, 30, 42–49.
- Carod-Artal, F.J.; Vazquez-Cabrera, C.B. Mescaline and the San Pedro cactus ritual: Archaeological and ethnographic evidence in northern Peru. Rev. Neurol. 2006, 42, 489–498.
- Castro-Klaren, S. Chavin: How Do We Understand Thee? An Inquiry into the Jaguar and the San Pedro Cactus. Rev. Estud. Hisp. 2021, 55, 515–535.
- Diaz, J.L. Sacred plants and visionary consciousness. Phenomenol. Cogn. Sci. 2010, 9, 159–170.
- Vamvakopoulou, I.A.; Narine, K.A.D.; Campbell, I.; Dyck, J.R.B.; Nutt, D.J. Mescaline: The forgotten psychedelic. Neuropharmacology 2023, 222, 109294.
- Dinis-Oliveira, R.J.; Pereira, C.L.; da Silva, D.D. Pharmacokinetic and Pharmacodynamic Aspects of Peyote and Mescaline: Clinical and Forensic Repercussions. Curr. Mol. Pharm. 2019, 12, 184–194.
- Dell’Erba, S.; Brown, D.J.; Proulx, M.J. Synesthetic hallucinations induced by psychedelic drugs in a congenitally blind man. Conscious Cogn. 2018, 60, 127–132.
- Kolaczynska, K.E.; Luethi, D.; Trachsel, D.; Hoener, M.C.; Liechti, M.E. Receptor Interaction Profiles of 4-Alkoxy-3,5-Dimethoxy-Phenethylamines (Mescaline Derivatives) and Related Amphetamines. Front. Pharmacol. 2022, 12, 794254.
- Cumming, P.; Scheidegger, M.; Dornbierer, D.; Palner, M.; Quednow, B.B.; Martin-Soelch, C. Molecular and Functional Imaging Studies of Psychedelic Drug Action in Animals and Humans. Molecules 2021, 26, 2451.
- Lopez-Gimenez, J.F.; Gonzalez-Maeso, J. Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. Behav. Neurobiol. Psychedelic Drugs 2018, 36, 45–73.
- McLean, T.H.; Chambers, J.J.; Parrish, J.C.; Braden, M.R.; Marona-Lewicka, D.; Kurrasch-Orbaugh, D.; Nichols, D.E. C-(4,5,6-Trimethoxyindan-1-yl)methanamine: A Mescaline Analogue Designed Using a Homology Model of the 5-HT2A Receptor. J. Med. Chem. 2006, 49, 4269–4274.
- Yamamoto, T.; Ueki, S. Behavioral-Effects of 2,5-Dimethoxy-4-Methylamphetamine (Dom) in Rats and Mice. Eur. J. Pharm. 1975, 32, 156–162.
- Halberstadt, A.L.; Geyer, M.A. Effect of Hallucinogens on Unconditioned Behavior. Curr. Top Behav. Neuro 2018, 36, 159–199.
- Halberstadt, A.L.; Powell, S.B.; Geyer, M.A. Role of the 5-HT2A receptor in the locomotor hyperactivity produced by phenylalkylamine hallucinogens in mice. Neuropharmacology 2013, 70, 218–227.
- Geyer, M.A.; Petersen, L.R.; Rose, G.J.; Horwitt, D.D.; Light, R.K.; Adams, L.M.; Zook, J.A.; Hawkins, R.L.; Mandell, A.J. The effects of lysergic acid diethylamide and mescaline-derived hallucinogens on sensory-integrative function: Tactile startle. J. Pharmacol. Exp. Ther. 1978, 207, 837–847.
- Trevizan, R.; Ludtke, R.; Cardoso, J.C.F.; Oliveira, P.E.; Streher, N.S. Is distyly in subtropical Psychotria brachyceras (Rubiaceae) similar to the general trends observed for the genus? Acta Bot. Bras. 2021, 35, 627–637.
- Lachenaud, O. Diversity and endemism of Psychotria in West and Central Africa. Opera Bot Belg 2019, 17, 71–84.
- Lachenaud, O. Revision of the genus Psychotria (Rubiaceae) in West and Central Africa General context of the study. Opera Bot Belg 2019, 17, 15.
- Lachenaud, O. Morphological characteristics of the genus. Opera Bot Belg 2019, 17, 33–59.
- Lachenaud, O. Taxonomic revision of the genus. Opera Bot Belg 2019, 17, 95–399.
- Lachenaud, O. Ecology. Opera Bot Belg 2019, 17, 61–70.
- Lachenaud, O. Revision du Genre Psychotria (Rubiaceae) en Afrique Occidentale et Centrale, Tomes 1&2. Opera Bot Belg 2019, 17, 1–909.
- Lachenaud, O. Local names and uses. Opera Bot Belg 2019, 17, 87–93.
- Soares, D.B.S.; Duarte, L.P.; Cavalcanti, A.D.; Silva, F.C.; Braga, A.D.; Lopes, M.T.P.; Takahashi, J.A.; Vieira, S.A. Psychotria viridis: Chemical constituents from leaves and biological properties. Acad. Bras. Cienc. 2017, 89, 927–938.
- Barker, S.A. N, N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function. Front. Neurosci. 2018, 12, 536.
- Pic-Taylor, A.; da Motta, L.G.; de Morais, J.A.; Melo, W.; Santos, A.D.A.; Campos, L.A.; Mortari, M.R.; von Zuben, M.V.; Caldas, E.D. Behavioural and neurotoxic effects of ayahuasca infusion (Banisteriopsis caapi and Psychotria viridis) in female Wistar rat. Behav. Process. 2015, 118, 102–110.
- James, E.; Keppler, J.; Robertshaw, T.L.; Sessa, B. N,N-dimethyltryptamine and Amazonian ayahuasca plant medicine. Hum. Psychopharmacol. Clin. Exp. 2022, 37, e2835.
- Serra, Y.A.; Barros-Santos, T.; Anjos-Santos, A.; Kisaki, N.D.; Jovita-Farias, C.; Leite, J.P.C.; Santana, M.C.E.; Coimbra, J.P.S.A.; de Jesus, N.M.S.; Sulima, A.; et al. Role of 5-HT2A receptors in the effects of ayahuasca on ethanol self-administration using a two-bottle choice paradigm in male mice. Psychopharmacology 2022, 239, 1679–1687.
- Gerwe, H.; He, F.; Pottie, E.; Stove, C.; Decker, M. Enlightening the “Spirit Molecule”: Photomodulation of the 5-HT2A Receptor by a Light-Controllable N,N-Dimethyltryptamine Derivative. Angew. Chem. Int. Ed. 2022, 61, e202203034.
- Rossi, G.N.; Guerra, L.T.L.; Baker, G.B.; Dursun, S.M.; Saiz, J.C.B.; Hallak, J.E.C.; dos Santos, R.G. Molecular Pathways of the Therapeutic Effects of Ayahuasca, a Botanical Psychedelic and Potential Rapid-Acting Antidepressant. Biomolecules 2022, 12, 1618.
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937.
- Inserra, A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front. Pharm. 2018, 9, 330.
- Nemes, B.; Peto, K.; Nemeth, N.; Mester, A.; Magyar, Z.; Ghanem, S.; Sogor, V.; Tanczos, B.; Deak, A.; Kallay, M.; et al. N,N-dimethyltryptamine Prevents Renal Ischemia-Reperfusion Injury in a Rat Model. Transplant. Proc. 2019, 51, 1268–1275.
- Su, T.P.; Hayashi, T.; Vaupel, D.B. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci. Signal. 2009, 2, pe12.
- Abatematteo, F.S.; Niso, M.; Contino, M.; Leopoldo, M.; Abate, C. Multi-Target Directed Ligands (MTDLs) Binding the sigma(1) Receptor as Promising Therapeutics: State of the Art and Perspectives. Int. J. Mol. Sci. 2021, 22, 6359.
- Penke, B.; Fulop, L.; Szucs, M.; Frecska, E. The Role of Sigma-1 Receptor, an Intracellular Chaperone in Neurodegenerative Diseases. Curr. Neuropharmacol. 2018, 16, 97–116.
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862.
- Szabo, I.; Varga, V.E.; Dvoracsko, S.; Farkas, A.E.; Kormoczi, T.; Berkecz, R.; Kecskes, S.; Menyhart, A.; Frank, R.; Hantosi, D.; et al. N,N-Dimethyltryptamine attenuates spreading depolarization and restrains neurodegeneration by sigma-1 receptor activation in the ischemic rat brain. Neuropharmacology 2021, 192, 108612.
- Barbano, A.C.; der Mei, W.F.; deRoon-Cassini, T.A.; Grauer, E.; Lowe, S.R.; Matsuoka, Y.J.; O’Donnell, M.; Olff, M.; Qi, W.; Ratanatharathorn, A.; et al. Differentiating PTSD from anxiety and depression: Lessons from the ICD-11 PTSD diagnostic criteria. Depress. Anxiety 2019, 36, 490–498.
- Frecska, E.; Szabo, A.; Winkelman, M.J.; Luna, L.E.; McKenna, D.J. A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. J. Neural. Transm. 2013, 120, 1295–1303.
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The Endogenous Hallucinogen and Trace Amine N,N-Dimethyltryptamine (DMT) Displays Potent Protective Effects against Hypoxia via Sigma-1 Receptor Activation in Human Primary iPSC-Derived Cortical Neurons and Microglia-Like Immune Cells. Front. Neurosci. 2016, 10, 423.
- Morales-Garcia, J.A.; Calleja-Conde, J.; Lopez-Moreno, J.A.; Alonso-Gil, S.; Sanz-SanCristobal, M.; Riba, J.; Perez-Castillo, A. N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo. Transl. Psychiatry 2020, 10, 1–14.
- Lie, D.C.; Song, H.J.; Colamarino, S.A.; Ming, G.L.; Gage, F.H. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu. Rev. Pharm. 2004, 44, 399–421.
- Cummings, J. Disease modification and Neuroprotection in neurodegenerative disorders. Transl. Neurodegener. 2017, 6, 1–7.
- Lauterbach, E.C.; Victoroff, J.; Coburn, K.L.; Shillcutt, S.D.; Doonan, S.M.; Mendez, M.F. Psychopharmacological Neuroprotection in Neurodegenerative Disease: Assessing the Preclinical Data. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 8–18.
- Apple, D.M.; Fonseca, R.S.; Kokovay, E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017, 1655, 270–276.
- Davis, A.K.; So, S.; Lancelotta, R.; Barsuglia, J.P.; Griffiths, R.R. 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am. J. Drug Alcohol Abuse 2019, 45, 161–169.
- thaug, M.V.; Lancelotta, R.; van Oorsouw, K.; Kuypers, K.P.C.; Mason, N.; Rak, J.; Šuláková, A.; Jurok, R.; Maryška, M.; Kuchař, M.; et al. A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology 2019, 236, 2653–2666.
- Michael, P.; Luke, D.; Robinson, O. An Encounter With the Other: A Thematic and Content Analysis of DMT Experiences From a Naturalistic Field Study. Front. Psychol. 2021, 12, 720717.
- Jimenez-Garrido, D.F.; Gomez-Sousa, M.; Ona, G.; Dos Santos, R.G.; Hallak, J.E.C.; Alcazar-Corcoles, M.A.; Bouso, J.C. Effects of ayahuasca on mental health and quality of life in naive users: A longitudinal and cross-sectional study combination. Sci. Rep. 2020, 10, 4075.
- Lee, J.W.; Park, M.S.; Park, J.-H.; Cho, Y.; Kim, C.; Kim, C.S.; Jo, J.W.; Lim, Y.W. Taxonomic Study of the Genus Pholiota (Strophariaceae, Basidiomycota) in Korea. Mycobiology 2020, 48, 476–483.
- Dorner, S.; Rogge, K.; Fricke, J.; Schafer, T.; Wurlitzer, J.M.; Gressler, M.; Pham, D.N.K.; Manke, D.R.; Chadeayne, A.R.; Hoffmeister, D. Genetic Survey of Psilocybe Natural Products. Chembiochem 2022, 23, e202200249.
- Van Court, R.C.; Wiseman, M.S.; Meyer, K.W.; Ballhorn, D.J.; Amses, K.R.; Slot, J.C.; Dentinger, B.T.M.; Garibay-Orijel, R.; Uehling, J.K. Diversity, biology, and history of psilocybin-containing fungi: Suggestions for research and technological development. Fungal Biol. 2022, 126, 308–319.
- Akers, B.P.; Ruiz, J.F.; Piper, A.; Ruck, C.A.P. A Prehistoric Mural in Spain Depicting Neurotropic Psilocybe Mushrooms? 1. Econ. Bot. 2011, 65, 121–128.
- Klein, A.K.; Chatha, M.; Laskowski, L.J.; Anderson, E.I.; Brandt, S.D.; Chapman, S.J.; McCorvy, J.D.; Halberstadt, A.L. Investigation of the Structure-Activity Relationships of Psilocybin Analogues. Acs Pharm. Transl 2021, 4, 533–542.
- Kolaczynska, K.E.; Liechti, M.E.; Duthaler, U. Development and validation of an LC-MS/MS method for the bioanalysis of psilocybin’s main metabolites, psilocin and 4-hydroxyindole-3-acetic acid, in human plasma. J. Chromatogr. B 2020, 1164, 122486.
- Brown, R.T.; Nicholas, C.R.; Cozzi, N.V.; Gassman, M.C.; Cooper, K.M.; Muller, D.; Thomas, C.D.; Hetzel, S.J.; Henriquez, K.M.; Ribaudo, A.S.; et al. Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clin. Pharm. 2017, 56, 1543–1554.
- Erkizia-Santamaria, I.; Alles-Pascual, R.; Horrillo, I.; Meana, J.J.; Ortega, J.E. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed Pharm. 2022, 154, 113612.
- Odland, A.U.; Kristensen, J.L.; Andreasen, J.T. Investigating the role of 5-HT2A and 5-HT2C receptor activation in the effects of psilocybin, DOI, and citalopram on marble burying in mice. Behav. Brain Res. 2020, 401, 113093.
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, N.F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835.
- Glatfelter, G.C.; Pottie, E.; Partilla, J.S.; Sherwood, A.M.; Kaylo, K.; Pham, D.N.K.; Naeem, M.; Sammeta, V.R.; DeBoer, S.; Golen, J.A.; et al. Structure–Activity Relationships for Psilocybin, Baeocystin, Aeruginascin, and Related Analogues to Produce Pharmacological Effects in Mice. ACS Pharm. Transl. 2022, 5, 1181–1196.
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411.
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The Therapeutic Potential of Psilocybin. Molecules 2021, 26, 2948.
- Sakashita, Y.; Abe, K.; Katagiri, N.; Kambe, T.; Saitoh, T.; Utsunomiya, I.; Horiguchi, Y.; Taguchi, K. Effect of psilocin on extracellular dopamine and serotonin levels in the mesoaccumbens and mesocortical pathway in awake rats. Biol. Pharm. Bull. 2015, 38, 134–138.
- Cao, C.; Barros-Álvarez, X.; Zhang, S.; Kim, K.; Dämgen, M.A.; Panova, O.; Suomivuori, C.-M.; Fay, J.F.; Zhong, X.; Krumm, B.E.; et al. Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 2022, 110, 3154–3167.e3157.
- Cao, D.; Yu, J.; Wang, H.; Luo, Z.; Liu, X.; He, L.; Qi, J.; Fan, L.; Tang, L.; Chen, Z.; et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022, 375, 403–411.
- Gumpper, R.H.; Fay, J.F.; Roth, B.L. Molecular insights into the regulation of constitutive activity by RNA editing of 5HT2C serotonin receptors. Cell Rep. 2022, 40, 111211.
- Studerus, E.; Kometer, M.; Hasler, F.; Vollenweider, F.X. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J. Psychopharmacol. 2011, 25, 1434–1452.
- Gukasyan, N.; Davis, A.K.; Barrett, F.S.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Griffiths, R.R. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J. Psychopharmacol. 2022, 36, 151–158.
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648.
- Zeiss, R.; Gahr, M.; Graf, H. Rediscovering Psilocybin as an Antidepressive Treatment Strategy. Pharmaceuticals 2021, 14, 985.
- Abate, G.; Uberti, D.; Tambaro, S. Potential and Limits of Cannabinoids in Alzheimer’s Disease Therapy. Biology 2021, 10, 542.
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased global integration in the brain after psilocybin therapy for depression. Nat. Med. 2022, 28, 844–851.
- Oberti, H.; Dalla Rizza, M.; Reyno, R.; Murchio, S.; Altier, N.; Abreo, E. Diversity of Claviceps paspali reveals unknown lineages and unique alkaloid genotypes. Mycologia 2020, 112, 230–243.
- Hradilov, M.; Vrabka, J.; Vankov, V.; Galuszka, P. Ergot alkaloid production in Claviceps purpurea is regulated by tryptophan related genes. New Biotechnol. 2018, 44, S115.
- Tasker, N.R.; Wipf, P. Biosynthesis, total synthesis, and biological profiles of Ergot alkaloids. Alkaloids. Chem. Biol. 2021, 85, 1–112.
- Hofmann, A. History of the Discovery of Lsd. 50 Years Lsd 1994, 7–16.
- Holloway, K. The Secret Black History of LSD. Nation 2022, 314, 16–19.
- Bayne, T.; Carter, O. Dimensions of consciousness and the psychedelic state. Neurosci Conscious 2018, 4, niy008.
- Juszczak, G.R. Disrupted integration of sensory stimuli with information about the movement of the body as a mechanism explaining LSD-induced experience. Med. Hypotheses 2017, 100, 94–97.
- Preller, K.H.; Vollenweider, F.X. Phenomenology, Structure, and Dynamic of Psychedelic States. Curr. Top. Behav. Neuro. 2018, 36, 221–256.
- Timmermann, C.; Spriggs, M.J.; Kaelen, M.; Leech, R.; Nutt, D.J.; Moran, R.J.; Carhart-Harris, R.L.; Muthukumaraswamy, S.D. LSD modulates effective connectivity and neural adaptation mechanisms in an auditory oddball paradigm. Neuropharmacology 2018, 142, 251–262.
- Family, N.; Hendricks, P.S.; Williams, L.T.J.; Luke, D.; Krediet, E.; Maillet, E.L.; Raz, S. Safety, tolerability, pharmacokinetics, and subjective effects of 50, 75, and 100 mu g LSD in healthy participants within a novel intervention paradigm: A proof-of-concept study. J. Psychopharmacol. 2022, 36, 321–336.
- Family, N.; Maillet, E.L.; Williams, L.T.J.; Krediet, E.; Carhart-Harris, R.L.; Williams, T.M.; Nichols, C.D.; Goble, D.J.; Raz, S. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology 2020, 237, 841–853.
- Grumann, C.; Henkel, K.; Brandt, S.D.; Stratford, A.; Passie, T.; Auwarter, V. Pharmacokinetics and subjective effects of 1P-LSD in humans after oral and intravenous administration. Drug Test Anal. 2020, 12, 1144–1153.
- Holze, F.; Duthaler, U.; Vizeli, P.; Liechti, M.E. Pharmacokinetics of an oral lysergic acid diethylamide (LSD) solution in healthy subjects. Clin. Toxicol. 2019, 57, 531.
- Liechti, M.E.; Schmid, Y.; Rentsch, K.M.; Hammann, F.; Dolder, P.C. Pharmacokinetics and pharmacodynamics of two doses of oral LSD in healthy subjects. Clin. Toxicol. 2017, 55, 475.
- Libânio Osório Marta, R.F. Metabolism of lysergic acid diethylamide (LSD): An update. Drug Metab. Rev. 2019, 51, 378–387.
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The pharmacology of lysergic acid diethylamide: A review. CNS Neurosci. Ther. 2008, 14, 295–314.
- Wacker, D.; Wang, S.; McCorvy, J.D.; Betz, R.M.; Venkatakrishnan, A.J.; Levit, A.; Lansu, K.; Schools, Z.L.; Che, T.; Nichols, D.E.; et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 2017, 168, 377–389.e312.
- Wang, C.; Jiang, Y.; Ma, J.; Wu, H.; Wacker, D.; Katritch, V.; Han, G.W.; Liu, W.; Huang, X.-P.; Vardy, E.; et al. Structural Basis for Molecular Recognition at Serotonin Receptors. Science 2013, 340, 610–614.
- De Gregorio, D.; Popic, J.; Enns, J.P.; Inserra, A.; Skalecka, A.; Markopoulos, A.; Posa, L.; Lopez-Canul, M.; Qianzi, H.; Lafferty, C.K.; et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2021, 118, e2020705118.
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182.
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci. 2020, 11, 864–871.
