The intestinal microbiota consists of trillions of bacteria, viruses, and fungi that achieve a perfect symbiosis with the host. They perform immunological, metabolic, and endocrine functions in the body. The microbiota is formed intrauterine. Dysbiosis is a microbiome disorder characterized by an imbalance in the composition of the microbiota, as well as changes in their functional and metabolic activities. The causes of dysbiosis include improper nutrition in pregnant women, hormone therapy, the use of drugs, especially antibiotics, and a lack of exposure to the mother’s vaginal microbiota during natural birth. Changes in the intestinal microbiota are increasingly being identified in various diseases, starting in the early neonatal period into the adult period. It has become more and more obvious that the components of the intestinal microbiota are crucial for the proper development of the immune system, and its disruption leads to disease.
1. Introduction
The human microbiota consists of different types of microorganisms that include bacteria, viruses, and fungi. They achieve symbiosis with the host and have important metabolic, immunological, and endocrine functions in the human body. The composition of the gut microbiota differs between individuals, varies during growth, and depends on host-environment interactions. The gut microbiota is constantly exposed to various external influences
[1]. In the intestinal tract, bacteria, viruses, and fungi are in dynamic balance. The existence of viruses as part of the intestinal microbiome has been neglected for a long time; 90% of them are in the form of bacteriophages, and approximately 10% of viruses are plant or animal viruses that are ingested with food. A community of bacteria, viruses, and fungi colonize the intestinal tract before birth, contributing to nutrient metabolism, stimulating the immune system, and protecting the host from pathogens
[2].
The formation and multiplication of the gut microbiome start from birth, while the modification of their composition depends mainly on various genetic, nutritional, and environmental factors. The primary obstacle to the proper development of intestinal microbiota is improper nutrition (excessive consumption of processed food of animal origin, insufficient intake of fresh fruit and especially vegetables, as well as vegetable juices, unnecessary overuse of antibiotics, and insufficient intake of breast milk or a short period of breastfeeding), which increases the inflammatory potential of intestinal microbiota
[3][6]. The microbiota of the intestine, vagina, and breast milk influence the colonization of newborns. The composition of the mother’s gut before delivery affects the creation of fetal immunity. Although the function of the placental microbiome is not clear, it is known that the gut microbiota during pregnancy is the most important factor in the health of the offspring
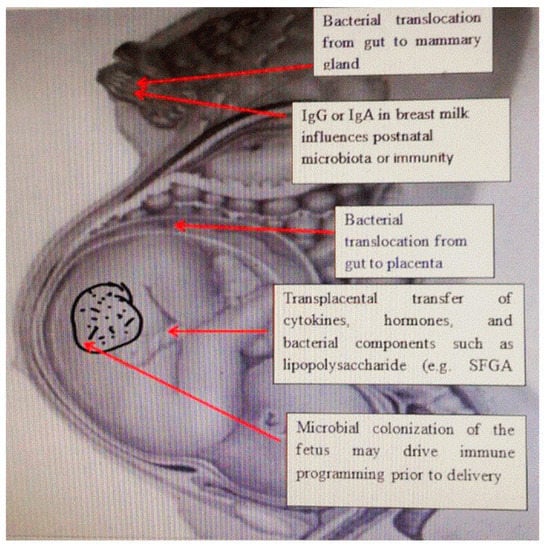
[4][7]. The passage of bacteria from the intestines of pregnant women and its colonization in extraintestinal places during pregnancy explains the presence of bacteria in breast milk. The transfer of antibodies through breastfeeding provides immune protection to the newborn; however, IgG-related bacteria can also be transferred after birth, as well as the transplacental IgG-mediated transfer of bacteria that can affect the developing immune system. This suggests that fetal immune programming in utero depends in part on the IgG-mediated transfer of bacterial components. Transplacental immune regulation can also be mediated by cytokines and hormones, as well as bacterial components such as lipopolysaccharides
[5][8] (
Figure 1).
Figure 1. The relationship between maternal microbiota and newborn immunity. Commensal microbes from the intestine of the pregnant woman, and the placenta and mammary glands affect the development of immunity in the fetus and the newborn by releasing short-chain fatty acids, and antibodies and changing the cytokine environment.
2. Intestinal Microbiome of Newborns
The gastrointestinal tract (GI) is undoubtedly the largest and most important organ of the immune system in the body, having a central role in the immune response of the organism. The intestinal epithelial barrier is in constant interaction with the intestinal microbiome and the cells of the immune system. The communication between epithelial cells, immune cells, and the microbiome is the most important as the specific immune responses to antigens depend on this communication. A change in the intestinal microorganisms (dysbiosis) induces the immune response and increases the body’s sensitivity to most diseases in early and adult life
[6][9].
Upon birth, the intestinal microbiota of the newborn is dominated by the genera
Enterobacteriaceae and
Staphylococcus. Following that, lactic acid bacteria and
Bifidobacterium are dominant. Bacteria from the genus
Bifidobacterium prevail until the introduction of solid food. After the weaning period, the
Bacteroides, Prevotella, Ruminococcus, Clostridium, and
Veillonella genera dominate. The microbiota prior to the weaning period is rich in the bacteria that facilitate the use of lactate, while after the weaning period, solid food promotes the growth of bacteria that contain genes that code for carbohydrate breakdown, vitamin synthesis, and xenobiotic degradation
[7][10]. The type of birth is one of the factors that influence the development of the microbiota in early childhood. In children born vaginally, the gut microbiota is similar to the mother’s vaginal microbiota, where
Lactobacillus, Prevotella, and
Sneathia genera predominate, while the gut microbiota of children born by cesarean section is similar to the skin microbiota, where
Staphylococcus, Corynebacterium and
Propionibacterium genera predominate. Some studies have shown that the colonization by the
Bacteroides and
Bifidobacterium genera starts one month after birth, while the concentration of
Clostridium difficile is very high. A decrease in the presence of Bacteroides after three to four months in children born by cesarean section has also been reported, and the microbiota of these children was less diverse
[8][11].
The gut microbiota develops intensively from the second to the third year of life. After the third year of life, it is similar to the microbiota of an adult. The establishment of stable gut microbiota is accompanied by two important changes in childhood. The first occurs shortly after birth, during breastfeeding, when the dominant microbes are the genus Bifidobacterium. Another change occurs during the period of introducing solid food into the child’s diet. In this period, bacteria from the genus
Bacteroides and
Firmicutes dominate
[9][12].
A healthy intestinal flora is essential for the health of the host. The normal human microbiome consists of two bacterial phyla,
Bacteroidetes and
Firmicutes. The intestinal microbiota of neonates is developed by the end of the third year of life. The total number of bacteria is significantly lower in newborns than in adults and the elderly. In the fecal microbiota of newborns, bifidobacteria are the dominant microbial group. The number of
Firmicutes/Bacteroidetes increases in later life and continues to change with age
[10][19]. The
Firmicutes/Bacteroidetes ratio (F/B ratio) is an indicator of inflammatory bowel disease
[11][4]. Prematurely born children of pregnant women who received antibiotics have an increase in
Enterobacteriaceae (microorganisms belonging to the type
Proteobacteria), as well as a decrease in the amount of the
Bacteroidaceae family
[12][20].
The factors that change the intestinal microbiota are diverse and act in different periods of life. They include the method of delivery (vaginal or cesarean section); nutrition after birth (mother’s milk or adapted food); nutrition later in life (vegan, vegetarian, or diet including large quantities of meat or meat products); as well as antibiotics or molecules similar to the antibiotics that most often originate from food
[13][22]. The mother’s diet during pregnancy has a great influence on the formation of the intestinal microbiota of neonates. A diet rich in dietary fibers and proteins affects the creation of short-chain fatty acids (SCFA), which are known to be the main metabolites produced by the gut microbiota
[14][23].
Short-chain fatty acids play a role in the development of intestinal immunity by stimulating the development of memory CD4+ and CD8+ lymphocyte T cells and preserving the integrity of the intestinal epithelium
[15][26]. In addition, bacterial metabolites are potentially transferred from the maternal gut during pregnancy to the mammary glands and may influence postnatal immune development during breastfeeding. Butyric acid stimulates the expression of brain-derived neurotrophic factor (BDNF), which is involved in neurogenesis
[16][27]. Propionic acid has an impact on brain development, cognition, and behavior
[17][28].
3. Consequences of the Use of Antibiotics on Diseases of Neonates and Adult Diseases
The effects of antibiotics on the host by changing the intestinal microbiome are enormous and have an impact on various functions of the body, including changes in the immune system and metabolic activities, creating the basis for the development of many diseases in both the neonatal and infant periods, as well as in adulthood
[18][52].
Trillions of bacteria inhabit the gastrointestinal (GI) tract, and the main role is played by commensals, which protect against the unwanted effects of pathogens.
Clostridium difficile is a bacterium that occurs most often in hospitalized patients treated with antibiotics in the form of long-term, debilitating diarrhea that causes dehydration of the patient. The therapy of choice is the transfer of healthy microbiota to treat patients with recurrent infections. In addition, commensal bacteria
C. scindens can inhibit the growth of
C. difficile by producing bile acids, deoxycholic acid (DCA), and lithocholic acid (LCA)
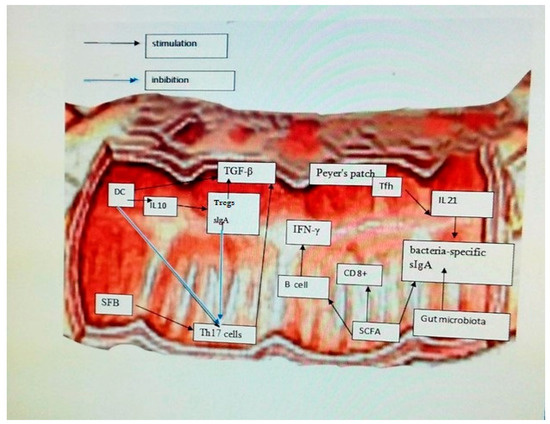
[19][38]. Dendritic cells (DC) respond to microbes by secreting cytokines that trigger inflammation and stimulate the adaptive immune response by producing Tregs and sIgA by secreting IL-10. Parts of bacteria, such as short-chain fatty acids (SCFA), induce T lymphocytes and B lymphocytes to produce gamma interferon (IFN-γ) from CD8+ T cells. Microbiota, SCFA, and IL-21 secreted from T follicular helper cells (Tfh) in Peyer’s patches (PP) contribute to the secretion of bacteria-specific sIgA. Sequestered fragments of bacteria (SFB) induce the production of Th17 cells. Tregs modulate the anti-inflammatory action of Transforming Growth Factor-β (TGF-β)-mediated DC and T helper 17 cells (Th17). DC and sIgA negatively regulate the pro-inflammatory function of Th17 cells by down-regulating synthesis 17 (IL17)
[20][53] (
Figure 2).
Figure 2. Effect of the modulatory role of intestinal microbiota and interactions between immune cells. Dendritic cells (DC) respond to microbes by secreting cytokines that trigger inflammation and stimulate the adaptive immune response by producing Tregs and sIgA by secreting IL-10. Parts of bacteria such as short-chain fatty acids (SCFA) induce T lymphocytes and B lymphocytes to produce gamma interferon (IFN-γ) from CD8+ T cells. Microbiota, SCFA, and IL-21 secreted from T follicular helper cells (Tfh) in Peyer’s patches (PP) contribute to the secretion of bacteria-specific sIgA. Sequestered fragments of bacteria (SFB) induce the production of Th17 cells. Tregs modulate the anti-inflammatory action of Transforming Growth Factor-β (TGF-β)-mediated DC and T helper 17 cells (Th17) e. DC and sIgA negatively regulate the pro-inflammatory function of Th17 cells by down-regulating synthesis 17 (IL17).
The pervasive developmental disorder includes a group of neurodevelopmental disorders of unknown etiology. Autism, which belongs to the group of pervasive developmental disorders, is the most common disorder from that group and appears no later than the third year of a child’s life. Genetic and environmental factors are considered to play an important role in the etiopathogenesis of the disease. This group of disorders is linked by characteristics such as difficulties in communication and social interaction and the presence of stereotyped actions. They are present in all patients and can be more or less pronounced. Children who have pervasive developmental disorders often also have symptoms of digestive system diseases, which indicates a possible role of the microbiota in the development of these disorders via the microbiota-intestine-brain axis. The increased permeability of the intestinal mucosa results in the presence of cytokines in the blood, such as interleukin-1b, interleukin-6, and interferon-g, but also parts of bacteria such as the lipopolysaccharide of gram-negative bacteria. By crossing the blood-brain barrier, they can activate an immune response in the brain and cause some of the behavioral symptoms of this spectrum of disorders. Differences in the microbiota of children with pervasive developmental disabilities compared to children without them exist. Children with this disorder have a less diverse microbiota, a smaller amount of
Bifidobacterium spp. and
Firmicutes spp., and higher levels of
Lactobacillus, Clostridium, and
Bacteroidetes. Despite this knowledge, a specific pattern of microbiota changes that could be used in the diagnosis or monitoring of individuals with pervasive developmental disorders has not yet been determined. Animal models have shown that mice born by cesarean section more often show signs of anxiety, worse social functioning, and repetitive actions, which has been confirmed by some human studies. In addition, their microbiota is disrupted and they show signs of increased intestinal permeability, which is a common comorbidity of pervasive developmental disorders
[21][56].
Allergic diseases are increasingly prevalent in children and can persist as a problem even in adulthood. Improvements in hygiene and changes in diet are considered to be the main cause of the increase in the frequency of allergic diseases. This hypothesis is called the “hygiene hypothesis” and is thought to also influence changes in the development of the microbiota. The microbiota affects the host’s immune response, including the development of the immune system at an early age, and because the pathogenesis of allergic diseases is directly related to the immune system, the role of the microbiota as a cofactor in the development of allergic diseases is obvious
[22][58]. Intestinal microorganisms, also in addition to all other microorganisms with which the newborn comes in contact, direct the development of this predominantly Th2 immune response into an immune response, which is more similar to adults and the maturation of regulatory T lymphocytes. This “immature” Th2 immune response causes an increased production of immunoglobulin E to various external antigens and the development of allergic reactions. By disrupting the normal development of the establishment of the intestinal microbiota, there is an increased risk for the development of allergic diseases, such as eczema, allergic rhinitis, and asthma. The use of antibiotics causes changes in the microbiota in children that create a predisposition to the development of allergic diseases. Taking antibiotics in the first year of life is associated with an increased frequency of asthma, as well as more severe forms. Stronger symptoms of rhinoconjunctivitis and eczema at ages six and seven are also associated with receiving antibiotics in infancy
[23][59]. Newborns born by caesarean section have a different intestinal microbiota compared to children born vaginally. It is interesting that elective caesarean section carries a higher risk of developing allergic diseases than emergency caesarean section, which is explained by the fact that, in most emergency caesarean sections, the rupture of the amniotic membrane occurs prior to the decision to have the procedure. It is believed that breastfeeding protects the child from the development of allergic diseases with its probiotic and prebiotic components
[24][60].