Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Pedro Alvarez.
The physicochemical features of the avian eggshell membrane play an essential role in the process of calcium carbonate deposition during shell mineralization, giving rise to a porous mineralized tissue with remarkable mechanical properties and biological functions. The membrane could be useful by itself or as a bi-dimensional scaffold to build future bone-regenerative materials.
- biopolymer
- bone biomaterial
- bone scaffold
- bone tissue engineering
1. Introduction
The avian eggshell membrane (ESM) is a versatile biomaterial with chemical characteristics and structural properties that could be exploited for bone regeneration [1]. It contains molecules of biomedical interest, such as collagen, hyaluronic acid, and dermatan sulfate [1]. Moreover, it has a mesh-type structure that behaves mechanically similarly to collagenous systems, such as tendons [1,2][1][2]. These properties resemble those of the collagenous matrix of bone, making the ESM a potential basis for bone scaffold development. Moreover, research for this ESM application constitutes an emerging subject that could be further amplified.
There is an ongoing demand for novel materials for bone regeneration due to the high prevalence of bone diseases. These diseases can lead to severe bone abnormalities and bone fragility, which in turn cause disability and decrease the quality of life [3,4,5,6][3][4][5][6]. Moreover, traumatic injury can require the replacement of damaged bone [7]. Currently, the most suitable material for bone regeneration is autologous bone. However, there are disadvantages and limitations regarding its extraction and possible post-operative complications after harvest [8,9][8][9]. Therefore, exploring the development of bone biomaterials with microstructure, biocompatibility, and bone-forming ability that overcome the limitations of autologous bone constitutes an active field of research [8]. Moreover, it is also essential to consider the cost-effectiveness of a biomaterial, which could lower the economic burden of high-priced biomedical materials [10].
Bone scaffolds are composite materials that can be fabricated using polymers, ceramics, or metals [11], as typified by a recently developed magnesium-based scaffold [12]. Polymers constitute reliable and versatile materials for fabricating bone scaffolds, primarily due to their broad biodegradation tunability, surface-to-volume ratio, heterogeneous porosity, and mechanical characteristics [13,14,15,16][13][14][15][16]. Polymeric materials also have considerable design potential arising from simple customization of their chemical and structural properties. Polymers can be synthetic or natural. Synthetic polymers have predictable properties and controllable synthesis [13,14][13][14]. However, they usually lack cell adhesion sites and are derived from nonrenewable resources [14,16][14][16]. Therefore, the sustainability of synthetic polymers is a potential drawback [16]. Some examples of these kinds of polymers are aliphatic polyesters, including poly-ε-caprolactone (PCL) and polylactic-based polyesters (PDLA, PLLA) [15]. Natural polymers are more sustainable and biodegradable than synthetic materials [13,14,16][13][14][16]. They possess molecules that are biomimetic, promote bioactivity, and support bone remodeling [15]. Consequently, it is worthwhile to investigate the properties of natural polymers to create bone biomaterials.
Moreover, biomaterials for bone regeneration based on natural polymers have great promise, since they are sustainable and contribute to a circular economy [17]. Biopolymers, notably cellulose, chitosan, and alginate, have been used as bone biomaterials [13,18,19][13][18][19]; however, they present shortcomings, including insufficient degradation [20], the presence of impurities [21], and limited long-term stability in physiological conditions [22]. The biodegradability, stability, and low immunogenicity of the ESM could compensate for the shortcomings of other biopolymers [23]. In addition, reusing this membrane requires little manufacturing, which exploits the advantages of its unique biological and mechanical properties [24]. Finally, research on the ESM for bone tissue engineering shows considerable potential due to the relative lack of studies associated with this application.
2. Eggshell Membrane Structure and Composition
The forming egg acquires its constituents as it passes through specialized regions of the avian oviduct [25]. In the white isthmus region, the tubular gland cells secrete the precursors of the ESM. The resulting fibers assemble into membranes that surround the rotating immature, uncalcified egg white while it traverses this region [26]. The membrane is progressively deposited as an interlaced fiber meshwork with difrenete morphological layers (Figure 1)
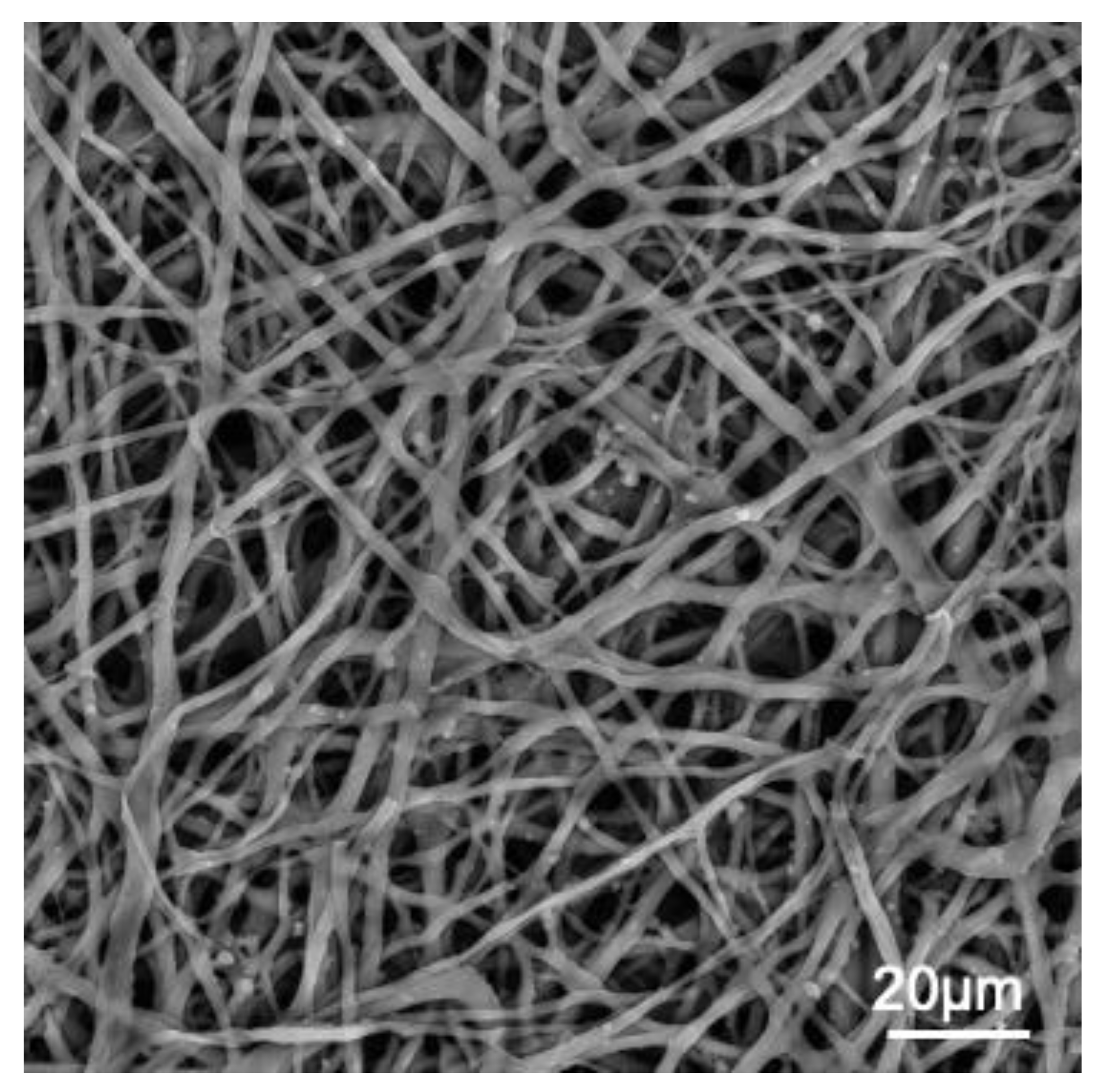
Figure 1.
SEM image of chicken ESM. scale bar ∼20 μm.
.The ESM consists predominantly of proteins (around 80–85%) [1]. However, it also contains CaCO3 mineral, sialic acid, uronic acid, and a minimal quantity of polysaccharides. In addition, small amounts of other ions, such as Mg, Si, and Zn, are also present [27][25]. The fibrous protein structure of the ESM is stabilized through extensive desmosine, isodesmosine, and disulfide cross-linkages, rendering it highly insoluble. Almost 500 proteins have been identified in the ESM proteome, which consists of structural proteins (collagens, CREMPs) as well as globular proteins (ovocalyxin−36, lysozyme, lysyl oxidase, etc.) [28][26]. Lysozyme is abundant in the inner and limiting membrane; moreover, purified egg white lysozyme can induce changes in calcite crystal morphology in vitro. The major structural protein is cysteine-rich eggshell membrane protein (abbreviated CREMP), but it also contains 10% collagens (collagens I, V, and predominantly X). The outer and inner membranes have collagens I and X, but only the inner membrane possesses type V collagen. According to estimates, the overall ratio between collagens I and V is 100:1 [29][27]. A small subset of proteins (#62) has been exclusively detected in the ESM of fertilized eggs at various stages of embryo development [30][28]. Comprehensive proteomic analyses of ESM have been performed [29][27]. The ESM possesses molecules similar to those of the bone matrix, namely type-I collagen in the fiber core and keratan sulfate in the mamillary knobs [1]. These molecules are essential in the nucleation processes of the shell mineral and might be useful for material development in bone tissue engineering.
The bulk elemental composition of the ESM is consistent, and no difference in the elemental composition of the different layers of the egg membrane has been detected. EDS analyses have detected C (~47 wt.%), H (~6 wt.%), N (~15–27 wt.%), O (~12–22 wt.%), S (3 wt.%), and Ca (~0.42 wt.%) [31][29].
The ATR-FTIR spectra of the manually obtained ESM display vibrational bands related to its organic components. The most intense bands are related to protein-bond vibrations (Table 1, Figure 2).
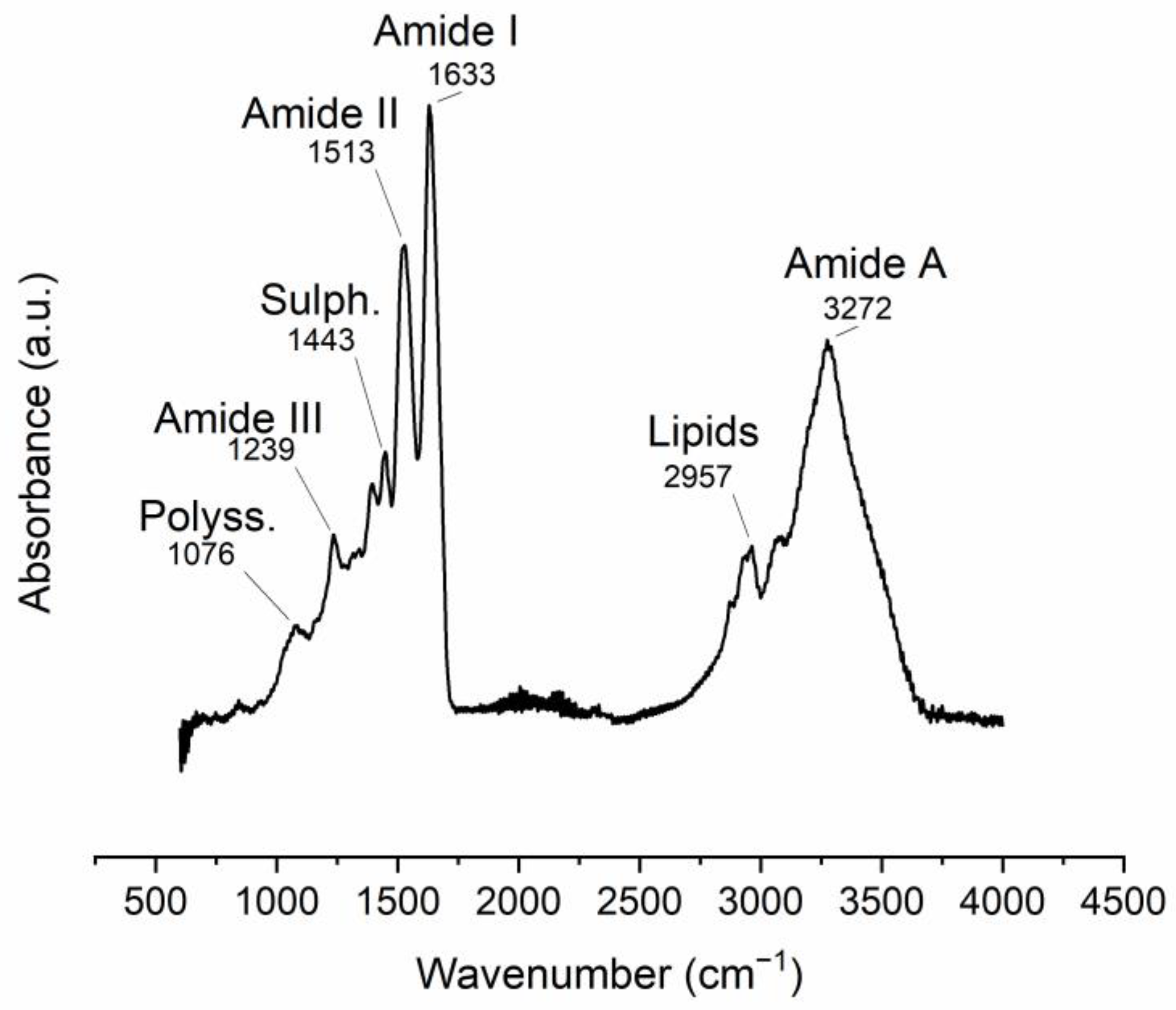
Figure 2. ATR-FTIR spectra of manually obtained ESM. Band position assignments are described in Table 1.
Table 1. FTIR vibrational bands observed in the ESM (m: medium, s: strong, v: very, w: weak).
| Band Position (cm−1) | Intensity | Vibration Description |
|---|---|---|
| 1076 | w | (Attributed to polysaccharides) |
| 1239 | w |
Table 2. Raman vibrational bands observed in the ESM (cm−1; m medium, s strong, v very, w weak).
| Band Position (cm−1) | Intensity | Vibration Description | |||
|---|---|---|---|---|---|
| 485 | vs | ν(S–S) stretching vibration | |||
| Amine C-N stretching (Amide III) | |||||
| 650 | m | Tyrosine and phenylalanine C-C twisting mode | 1443 | w | CH2 scissoring (attributed to sulfates) |
| 750 | m | Symmetric breathing of tryptophan | 1513 | s | C-N stretching/NH bending (Amide II) |
| 1010 | m | 1633 | vs | Amide C=O stretching (Amide I) | |
| 2426 | vw | Sulfhydryl group (-SH) 1 | |||
| 2957 | m | C-H stretching (attributed to lipids) | |||
| 3272 | S | O-H and N-H stretching (Amide A) |
1 possibly due to proteins rich in cysteine (i.e., CREMPS).
The ESM Raman spectrum displays bands that can be associated with protein structure. The 485 cm−1 band is attributed to the sulfur-containing proteins (cysteine/disulfide-rich; CREMPs) of the membranes (Figure 3, Table 2).
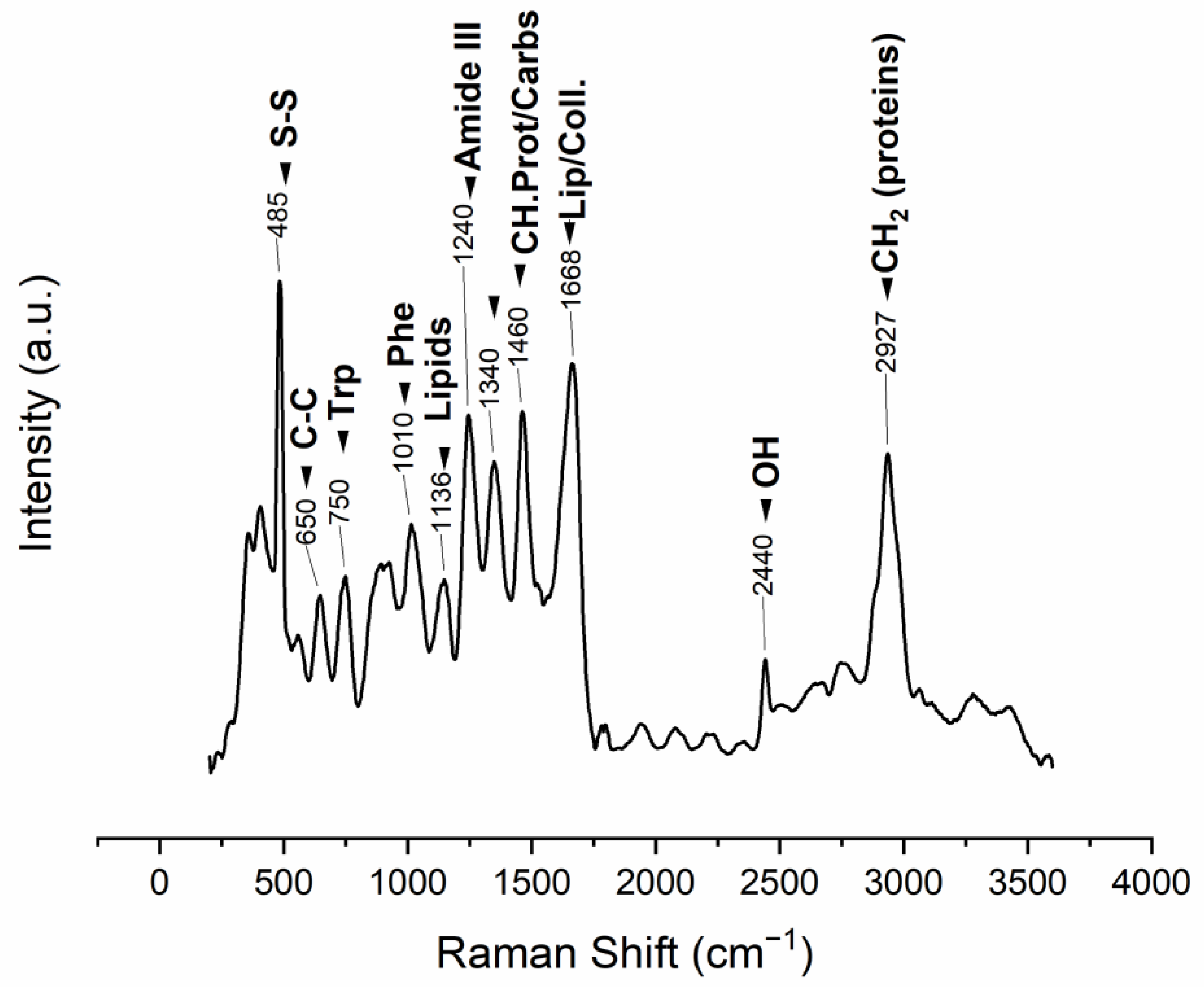
Figure 3.
Raman vibrational bands observed in the manually obtained ESM (m: medium, s: strong, v: very, w: weak), band position assignments in
Table 2
.
| Phenyl ring angular bending vibrations, related to phenylalanine | ||
| 1136 | ||
| s | Lipids | |
| 1240 | s | Amide III |
| 1340 | s | CH deformation (proteins and carbohydrates) |
| 1460 | s | CH2 wagging, CH2/CH3 deformation for lipids and collagen |
| 1668 | vs | Amide I |
| 2440 | w | OH stretching vibrations |
| 2927 | s | CH2 asymmetric stretch |
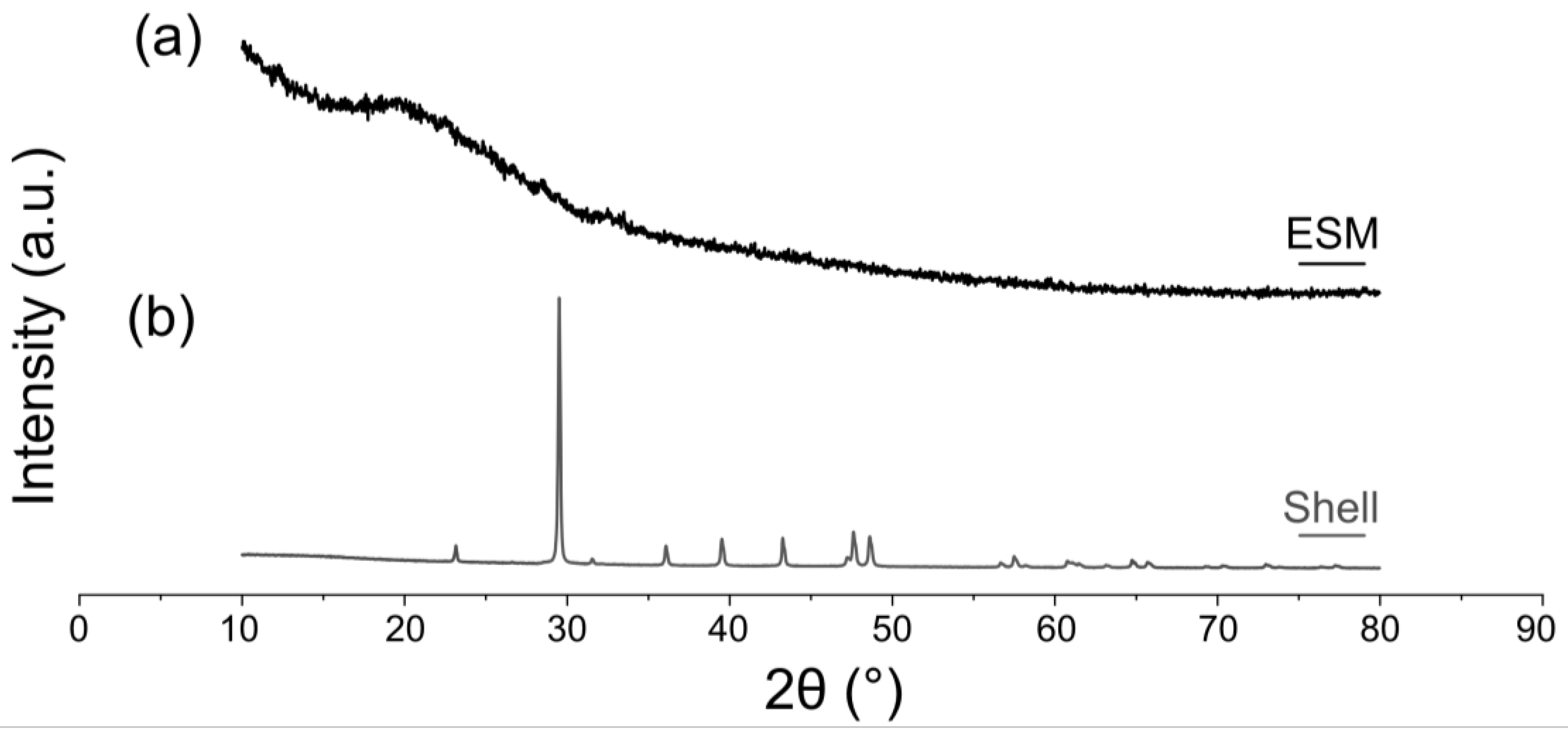
Figure 4. XRD patterns of the eggshell and ESM (mechanically removed from the shell). (a) ESM. (b) Mineral shell membrane without organic membrane. The membrane was removed manually.
References
- Baláž, M. Eggshell Membrane Biomaterial as a Platform for Applications in Materials Science. Acta Biomater. 2014, 10, 3827–3843. https://doi.org/10.1016/j.actbio.2014.03.020.
- Torres, F.G.; Troncoso, O.P.; Piaggio, F.; Hijar, A. Structure–Property Relationships of a Biopolymer Network: The Eggshell Membrane. Acta Biomater. 2010, 6, 3687–3693. https://doi.org/10.1016/j.actbio.2010.03.014.
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Sci. Eng. C 2021, 130, 112466. https://doi.org/10.1016/j.msec.2021.112466.
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. https://doi.org/10.1007/s00590-017-2063-0.
- de Villiers, T.J.; Goldstein, S.R. Bone Health 2022: An Update. Climacteric 2022, 25, 1–3. https://doi.org/10.1080/13697137.2021.1965408.
- Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Nagy, E.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; El-Husseini, A. Secondary Osteoporosis and Metabolic Bone Diseases. Clin. Med. 2022, 11, 2382. https://doi.org/10.3390/jcm11092382.
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Model. Mech. 2018, 11, 1–14. https://doi.org/10.1242/dmm.033084.
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Cell Dev. 2021, 9, 1–18. https://doi.org/10.3389/fcell.2021.665813.
- Riester, O.; Borgolte, M.; Csuk, R.; Deigner, H.-P. Challenges in Bone Tissue Regeneration: Stem Cell Therapy, Biofunctionality and Antimicrobial Properties of Novel Materials and Its Evolution. Int J. Mol. Sci 2020, 22, 192. https://doi.org/10.3390/ijms22010192.
- Tracy, A.A.; Bhatia, S.K.; Ramadurai, K.W. Impact of Biomaterials on Health and Economic Development. In Bio-Based Materials as Applicable; Accessible, and Affordable Healthcare Solutions; Tracy, A.A., Bhatia, S.K., Ramadurai, K.W., Eds.; SpringerBriefs in Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 33–41; ISBN 978-3-319-69326-2.
- Zhang, X.; Li, Q.; Wang, Z.; Zhou, W.; Zhang, L.; Liu, Y.; Xu, Z.; Li, Z.; Zhu, C.; Zhang, X. Bone regeneration materials and their application over 20 years: A bibliometric study and systematic review. Bioeng. Biotechnol. 2022, 10, 297. https://doi.org/10.3389/fbioe.2022.921092.
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. https://doi.org/10.3390/su15010823.
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric Biomaterials for Bone Regeneration. Jt. 2016, 1, 27. https://doi.org/10.21037/aoj.2016.11.02.
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. https://doi.org/10.3390/polym13071105.
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. https://doi.org/10.3390/polym12040905.
- Fouad, D.; Farag, M. Design for Sustainability with Biodegradable Composites. In Design and Manufacturing; IntechOpen: London, UK, 2019; pp. 1–20; ISBN 978-1-78985-866-2.
- Wojnarowska, M.; Sołtysik, M.; Guzik, M. Socio-Economic Importance of Biomaterials in the Transition to the Circular Economy Model. In Proceedings of the ; Volume 92, p. 05029. https://doi.org/10.1051/shsconf/20219205029.
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Zhu, Y.; Narayanan, K.B.; Han, S.S.; Park, J.K. Fabrication Strategies and Biomedical Applications of Three-Dimensional Bacterial Cellulose-Based Scaffolds: A Review. J. Biol. Macromol. 2022, 209, 9–30. https://doi.org/10.1016/j.ijbiomac.2022.03.191.
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958. https://doi.org/10.3390/polym14050958.
- Khan, S.; Siddique, R.; Huanfei, D.; Shereen, M.A.; Nabi, G.; Bai, Q.; Manan, S.; Xue, M.; Ullah, M.W.; Bowen, H. Perspective Applications and Associated Challenges of Using Nanocellulose in Treating Bone-Related Diseases. Bioeng. Biotechnol. 2021, 9, 616555. https://doi.org/10.3389/fbioe.2021.616555.
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Drugs 2016, 14, 99. https://doi.org/10.3390/md14050099.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Polym. Sci 2012, 37, 106–126. https://doi.org/10.1016/j.progpolymsci.2011.06.003.
- Dupoirieux, L.; Pourquier, D.; Picot, M.C.; Neves, M. Comparative Study of Three Different Membranes for Guided Bone Regeneration of Rat Cranial Defects. J. Oral Maxillofac. Surg. 2001, 30, 58–62. https://doi.org/10.1054/ijom.2000.0011.
- Baláž, M.; Boldyreva, E.V.; Rybin, D.; Pavlović, S.; Rodríguez-Padrón, D.; Mudrinić, T.; Luque, R. State-of-the-Art of Eggshell Waste in Materials Science: Recent Advances in Catalysis, Pharmaceutical Applications, and Mechanochemistry. Bioeng. Biotechnol. 2021, 8, 1522. https://doi.org/10.3389/fbioe.2020.612567.
- Tsai, W.; Yang, J.; Lai, C.; Cheng, Y.; Lin, C.; Yeh, C. Characterization and Adsorption Properties of Eggshells and Eggshell Membrane. Bioresour. Technol. 2006, 97, 488–493. https://doi.org/10.1016/j.biortech.2005.02.050.
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The Eggshell: Structure, Composition and Mineralization. Front. Biosci 2012, 17, 1266–1280. https://doi.org/10.2741/3985
- Mittal, A.; Teotia, M.; Soni, R.K.; Mittal, J. Applications of egg shell and egg shell membrane as adsorbents: A review. Mol. Liq. 2016, 223, 376–387. https://doi.org/10.1016/j.molliq.2016.08.065.
- Ahmed, T.A.E.; Suso, H.-P.; Hincke, M.T. In-Depth Comparative Analysis of the Chicken Eggshell Membrane Proteome. Proteom. 2017, 155, 49–62. https://doi.org/10.1016/j.jprot.2017.01.002.
- Carrino, D.A.; Dennis, J.E.; Wu, T.M.; Arias, J.L.; Fernandez, M.S.; Rodriguez, J.P.; Fink, D.J.; Heuer, A.H.; Caplan, A.I. The Avian Eggshell Extracellular Matrix as a Model for Biomineralization. Tissue Res. 1996, 35, 325–329.
- Wong, M.; Hendrix, M.J.; von der Mark, K.; Little, C.; Stern, R. Collagen in the Egg Shell Membranes of the Hen. Biol 1984, 104, 28–36. https://doi.org/10.1016/0012-1606(84)90033-2.
- Chen, X.; Zhu, L.; Wen, W.; Lu, L.; Luo, B.; Zhou, C. Biomimetic Mineralisation of Eggshell Membrane Featuring Natural Nanofiber Network Structure for Improving Its Osteogenic Activity. Colloids Surf. B Biointerfaces 2019, 179, 299–308. https://doi.org/10.1016/j.colsurfb.2019.04.009.
References
- Baláž, M. Eggshell Membrane Biomaterial as a Platform for Applications in Materials Science. Acta Biomater. 2014, 10, 3827–3843.
- Torres, F.G.; Troncoso, O.P.; Piaggio, F.; Hijar, A. Structure–Property Relationships of a Biopolymer Network: The Eggshell Membrane. Acta Biomater. 2010, 6, 3687–3693.
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C 2021, 130, 112466.
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362.
- de Villiers, T.J.; Goldstein, S.R. Bone Health 2022: An Update. Climacteric 2022, 25, 1–3.
- Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Nagy, E.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; El-Husseini, A. Secondary Osteoporosis and Metabolic Bone Diseases. J. Clin. Med. 2022, 11, 2382.
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Model. Mech. 2018, 11, dmm033084.
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. 2021, 9, 665813.
- Riester, O.; Borgolte, M.; Csuk, R.; Deigner, H.-P. Challenges in Bone Tissue Regeneration: Stem Cell Therapy, Biofunctionality and Antimicrobial Properties of Novel Materials and Its Evolution. Int J. Mol. Sci 2020, 22, 192.
- Tracy, A.A.; Bhatia, S.K.; Ramadurai, K.W. Impact of Biomaterials on Health and Economic Development. In Bio-Based Materials as Applicable; Accessible, and Affordable Healthcare Solutions; Tracy, A.A., Bhatia, S.K., Ramadurai, K.W., Eds.; SpringerBriefs in Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 33–41. ISBN 978-3-319-69326-2.
- Zhang, X.; Li, Q.; Wang, Z.; Zhou, W.; Zhang, L.; Liu, Y.; Xu, Z.; Li, Z.; Zhu, C.; Zhang, X. Bone regeneration materials and their application over 20 years: A bibliometric study and systematic review. Front. Bioeng. Biotechnol. 2022, 10, 297.
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823.
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric Biomaterials for Bone Regeneration. Ann. Jt. 2016, 1, 27.
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105.
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905.
- Fouad, D.; Farag, M. Design for Sustainability with Biodegradable Composites. In Design and Manufacturing; IntechOpen: London, UK, 2019; pp. 1–20. ISBN 978-1-78985-866-2.
- Wojnarowska, M.; Sołtysik, M.; Guzik, M. Socio-Economic Importance of Biomaterials in the Transition to the Circular Economy Model. In Proceedings of the 20th International Scientific Conference Globalization and its Socio-Economic Consequences 2020, Rajecke Teplice, Slovakia, 21–22 October 2020; Volume 92, p. 05029.
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Zhu, Y.; Narayanan, K.B.; Han, S.S.; Park, J.K. Fabrication Strategies and Biomedical Applications of Three-Dimensional Bacterial Cellulose-Based Scaffolds: A Review. Int. J. Biol. Macromol. 2022, 209, 9–30.
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958.
- Khan, S.; Siddique, R.; Huanfei, D.; Shereen, M.A.; Nabi, G.; Bai, Q.; Manan, S.; Xue, M.; Ullah, M.W.; Bowen, H. Perspective Applications and Associated Challenges of Using Nanocellulose in Treating Bone-Related Diseases. Front. Bioeng. Biotechnol. 2021, 9, 616555.
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci 2012, 37, 106–126.
- Dupoirieux, L.; Pourquier, D.; Picot, M.C.; Neves, M. Comparative Study of Three Different Membranes for Guided Bone Regeneration of Rat Cranial Defects. Int. J. Oral Maxillofac. Surg. 2001, 30, 58–62.
- Baláž, M.; Boldyreva, E.V.; Rybin, D.; Pavlović, S.; Rodríguez-Padrón, D.; Mudrinić, T.; Luque, R. State-of-the-Art of Eggshell Waste in Materials Science: Recent Advances in Catalysis, Pharmaceutical Applications, and Mechanochemistry. Front. Bioeng. Biotechnol. 2021, 8, 1522.
- Mittal, A.; Teotia, M.; Soni, R.K.; Mittal, J. Applications of egg shell and egg shell membrane as adsorbents: A review. J. Mol. Liq. 2016, 223, 376–387.
- Ahmed, T.A.E.; Suso, H.-P.; Hincke, M.T. In-Depth Comparative Analysis of the Chicken Eggshell Membrane Proteome. J. Proteom. 2017, 155, 49–62.
- Wong, M.; Hendrix, M.J.; von der Mark, K.; Little, C.; Stern, R. Collagen in the Egg Shell Membranes of the Hen. Dev. Biol. 1984, 104, 28–36.
- Kaweewong, K.; Garnjanagoonchorn, W.; Jirapakkul, W.; Roytrakul, S. Solubilization and Identification of Hen Eggshell Membrane Proteins During Different Times of Chicken Embryo Development Using the Proteomic Approach. Protein J. 2013, 32, 297–308.
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479.
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479.
More
