1. Biodegradable Microcarriers for Cell Manufacturing
Due to the limited amount of adult stem cells that can be retrieved from patients, it is necessary to generate large amounts of stem cells outside the human body, with a cost-effective approach. The use of microcarriers is an established technology in the biopharmaceutical industry, which, in combination with stirred-tank bioreactors, can provide the necessary environment for large-scale production of adherent cells. However, conventional microcarriers have been regarded as a potential safety risk to the patient, because particulates may remain in the final product. As such, traditional microcarriers have not been classified as cGMP compliant, which has hindered their widespread use in clinical trials or production processes for previously authorized autologous stem cell products
[1][31]. As a result, adult stem cells such as MSCs, even in clinical settings, are often still cultured in poorly controlled and labor-intensive two-dimensional tissue flasks. The development of microcarriers that can be dissolved in vitro, or degraded in vivo, could represent a major step forward in overcoming the existing challenges in stem cell expansion, and open opportunities for the use of volumetrically scalable bioreactors
[2][3][32,46]. In the case of dissolvable microcarriers, the cells could be harvested without the use of the traditional enzymatic dissociation method, by pH, temperature, biochemical changes in adherent molecules, changes in protein chemistry of surface receptors, and other biochemical changes that do not hamper the cells’ adherence features. Depending on the speed of degradation, cells growing on biodegradable microcarriers could be harvested by dissolving the microcarriers within the bioreactor, or both cells and microcarriers could directly be implanted into the site of injury
[4][47]. A recent report, showcased the use of porous PLGA microcarriers for the culturing of human adipose stem cells, which remained undifferentiated in dynamic culture conditions
[5][34]. Microcarriers were evaluated for stability at 37 °C, to cultivate cells, and found to be stable with no signs of degradation for up to two months in water, at 4 °C. The biodegradability and other bioengineering confirmation studies were reported in Muoio et al., which demonstrated the gradual degradability of the microcarriers under stirred conditions at 37 °C, when cultured for up to nine days
[6][33].
Likewise, for large-scale expansion of therapeutic cells, dispersible and dissolvable porous microcarrier material (3D TableTrix
TM) has been developed, and identified for use in stirred bioreactors
[7][35]. Briefly, 3D TableTrix
TM has been designed with a dispersible and dissolvable feature, that aids in avoiding the need for time-consuming microcarrier separation from cells, and its soluble property offers a higher rate of cell recovery. Authors have reported the potential use of this application in cell manufacturing, by showing 500-fold multiplication of adipose-derived mesenchymal stem cells (AdMSCs) in a 1 L bioreactor system, with a final cell yield of 1.05 ± 0.11 × 10
9 hMSCs, with 98.6% recovery rate in 11 days, cultured under serum-free conditions. Furthermore, cells maintained their differentiation abilities to trilineage, stable genomic profiles, as well as immunophenotypic profile, while exhibiting negligible signs of senescence.
Cultispher G, a cross-linked porous microcarrier, is commonly employed as a cell carrier in cell therapy applications. In stirred tank bioreactor culture, such gelatin-based microcarriers support a wide range of adherent cell types, and are scalable to hundreds of liters. Cultispher G is particularly beneficial, since it can be enzymatically dissolved, making cell harvesting easier, without the need for cell-bead filtering
[8][36]. The delayed destruction of deposited ECM, by enzymatic reagents, on the other hand, inhibits the cell recovery rate, decreasing cellular viability. The invention of the stimuli-triggered breakdown of cross-linked microcarriers for cell harvesting, has addressed these issues. In comparison to conventional beads, newly produced redox-sensitive beads (RS beads) have exhibited faster disintegration, allowing for greater hMSC dissociation, with significant cell yield after culturing for eight days
[9][37]. The concept has been tested and demonstrated in spinner flasks, as well as bioreactors. In comparison to Cultispher G beads, studies were conducted to ensure that surface modification of the microcarriers (RS beads) did not affect cell adhesion. After cell adhesion and growth in spinner flasks, the redox dissolving time for RS beads was found to be faster than the enzymatic dissociation time for conventional beads. MSCs grown on the RS beads did not show any significant difference in the growth curve, compared to the control regular beads. Interestingly, the cell harvest time in 3 L bioreactors, for cells cultured on RS beads, was at least 15 times more rapid than the control
[9][37]. RS beads show great potential as cell carriers in manufacturing applications, as they allow for cell proliferation with higher recovery yield.
Porous microcarriers are commonly used to grow, expand, and harvest stem cells. In most cases, the cells are harvested using proteolytic enzymes, which can result in cell damage. One of the studies developed a variety of alginate/PEG (AL/PEG) semi-interpenetrating network of microcarriers, to overcome such limitations. The interaction between the carboxylic acid group of alginate and the di-terminated amine groups of cystamine, was applied, to chemically cross-link alginate and PEG, to form networks. PEG was added to regulate the degradation of the microcarriers, and actively interact with the alginate network. Furthermore, the mechanical stability of the AL/PEG complex, was enhanced by the electrostatic characteristics of chitosan coated on the surface. Non-coated AL/PEG microcarriers exhibit poor mechanical stability, and this is worse when non-cross-linked PEG molecules are discharged into the culture medium. A chitosan coating was used to boost the mechanical stability of AL/PEG, and, as the authors expected, AL/PEG microcarriers with the chitosan coating had a greater cell proliferation rate, and after 5–7 days of culture, a 12-fold increase in cell yield was observed
[10][38]. The results revealed that PEG size and molecular weight modulated the microcarriers’ properties. Furthermore, the microcarriers were engineered to degrade when disulfide links were cleaved. The rate of microcarrier degradation was tuned, depending on changes in the AL to PEG ratio, the amount of chitosan coating, and the type and concentration of reductant utilized. AL/PEG microcarriers have also been developed to aid in the attachment and proliferation of MSCs. Therefore, a reductant overcame the constraints of cell harvesting from microcarriers, while also decreasing the cell damage induced by proteolytic enzyme treatment, and enhanced the cell yield.
2. Biodegradable Microcarriers for Tissue Engineering Applications
Scaffolds are a stable framework, that are made of polymeric biomaterials, which enable cells to bind onto the scaffold, to secrete ECM proteins that imitate the support of structures (the biophysical and biochemical indices of indigenous tissue in which cells can grow), to migrate, and eventually to transform into tissues
[11][12][39,40]. Substantial progress, and the development of advanced-engineered scaffold platforms, is needed for tissue repair applications, but growing large quantities of cells, ranging from millions to billions, with clinically amenable quality for therapies, remains a challenge to achieve
[13][14][41,43]. Due to a paucity of cells in cell banks for clinical infusion, an effective platform for the biomanufacturing of cellular products is needed, to meet clinical demand
[15][16][42,44].
Traditional tissue engineering approaches typically integrate three-dimensional (3D) scaffolds with cell sources and growth factors, to generate in vitro tissues. However, such tissue constructs have a history of failing to fill and heal irregularly shaped defects, such as cartilage replacement, thus restricting the clinical significance of tissue engineered products
[17][45]. To address such technical constraints, engineered microtissues, with cell-laden microcarriers, have been designed to precisely match defect areas, as building blocks for implantable/injectable treatment. After implantation, microcarriers embedded in tailored microtissues provide a critical frame for establishing functional tissue growth and anastomosis (i.e., connection between adjacent tissue structures). In a recent study, dialdehyde bacterial cellulose (DBC), a natural material with nanofibrous characteristics, was used to develop ECM-mimicking microcarriers, which could simulate the matrix complexity of collagen, hydroxylysine, and chitosan. Thus, replicating cartilage ECM, and potentially enhancing tissue repair and regeneration. The effects of several parameters, on the nanofibrous microcarriers, such as chitosan concentration, porosity, as well as biomechanical profile and degradation properties, have also been evaluated. The cytocompatibility was confirmed in vitro, by examining cell proliferation and viability.
The lack of biocompatible materials has hindered the advancement of biodegradable implants for bone tissue engineering. As a result, strengthening bioactivity through surface modification of the composite is critical for bone regeneration. BMP-2, a key component in initiating osteogenesis and facilitating bone repair, has been used extensively in clinical trials. Previous studies have found that the greater biodegradability of PLGA/HA nanocomposites, gives them higher biocompatibility and osteoconductivity properties for bone grafts. However, due to the polymers’ weak hydrophilicity and absence of functional groups, the growth factor loading efficiency is frequently reduced. Attempts were made to immobilize BMP-2 on graphene oxide (GO)-incorporated PLGA/HA (GO-PLGA/HA) biodegradable microcarriers. These biodegradable microcarriers also have the advantage of offering a substantial percentage of anchoring sites, which promotes cell adhesion. Chuan Fu et al. reported graphene oxide (GO)-promoted immobilization of peptides on PLGA/HA microcarriers, in less than 120 min; the cytocompatibility of MC3T3-E1 cells (murine cell line) cultivated on these microcarriers, resulted in significantly better cell adhesion and proliferation, via GO and HA
[3][46]. Furthermore, the π-electron clouds of GO are capable of interacting with the inner hydrophobic cores of BMP-2 protein, improving the protein adsorption capacity and efficiently increasing BMP-2 binding on the microcarrier surface, allowing microcarriers to perform long-term osteoconductivity. Immobilization of BMP-2 on GO-PLGA/HA microcarriers, enhanced osteogenic differentiation to a greater extent, which was confirmed by alkaline phosphate activity, qRT-PCR, immunofluorescence staining, and mineralization on the deposited substrates.
Bioprinting is the process of printing scaffolds with embedded cells, to fabricate tissue constructs for regenerative medicine applications. Although bioinks with cells improve biomimetic features, issues still exist with ECM formation, cell activity, proliferation, and the ability to change into functional tissue constructs that resemble native tissue
[18][19][51,52]. Although, bioprinting microcarriers seems a simple process, challenges, such as nozzle blockage, can arise when printing high cell densities. Microcarriers enable cells to self-assemble to high cell density within bioinks, and thus represent a favorable milieu for enhanced cell interaction, and fabrication of stable tissue constructs with more functional properties
[20][53]. Bioinks with porous biodegradable microcarriers embedded within hydrogels, have generated functional osteochondral tissue structures with high cell density, at 8 × 10
6/mL
[21][54]. Levato R. et al., reported 3D printing of MSCs in PLA microcarriers, which exhibited significantly greater inter-cellular interaction and differentiation potential compared to hydrogels with only cells, and no microcarrier controls
[20][53]. PLA microcarriers were pre-seeded with gelatin-methylacrylate and gellan gum (GelMA-GG) solution in one condition, whereas other PLA microcarriers were embedded with MSCs in GelMA-GG hydrogels for bioprinting. Cell viabilities of more than 90% have been reported after 3 days of culturing. Surprisingly, MSCs suspended with microcarriers in GelMA-GG hydrogels, attached to the surface of the microcarriers without the need for a pre-seeding step. The MSCs were observed to be at an early stage of adhesion onto the microcarriers after 4 h, in the presence of GelMA, whereas if seeded directly onto the microcarriers, they already expressed structured actin fibers
[20][53]. Thus, cell-laden biodegradable microcarriers for bioprinting and tissue engineering, could serve as essential modular components for 3D printing functional tissue structures.
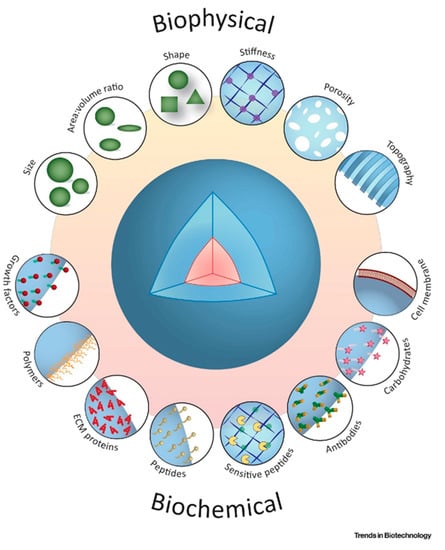
In the pursuit for a reliable and compliant cell expansion strategy, microcarriers with diverse physicochemical features have been designed. The shape and topographical features of microcarriers, such as interconnected pores, provide an expansive tissue-like microenvironment, that significantly improves cell growth and differentiation profiles
[22][55] as illustrated in
Figure 12. Engineered microcarriers can be configured to promote cell attachment and differentiation, and to be degradable at a controlled rate
[23][56]. Optimization across a wide range of cell densities is needed, to achieve implantable microcarrier populations for injection. Although their handling can be described as straight-forward, hydrogel-only injectable systems often have poor mechanical stability and are not sufficiently durable to support proliferation and differentiation of anchorage-dependent cells, before formation of new tissue
[24][57].
Figure 12. Schematic illustrations of biochemical and bio-physical indications contribute to the modulate topographical and architectural characteristics of biodegradable microcarriers for therapeutic applications. Adapted with permission from
[25][58].
3. Biodegradable Microcarriers for Drug Delivery
Microcarriers have sparked a surge of interest in drug delivery, as the production of functional carriers utilizes simple procedures with new, but accessible, materials. The development of smart, bioactive and biodegradable microcarriers, is important for enhancing drug delivery and promoting tissue repair, and personalized medicine as a clinical norm
[26][27][59,60]. Han Zhang et al.
, reported novel soybean protein microcarriers, using a microfluidic strategy for drug delivery, the technology was inspired by the tofu production mechanism, of combining soymilk and brine for cross-linking soybean proteins. Since soybean protein droplets are synthesized via a microfluidic emulsification method, tofu microcarriers are relatively monodispersed and have homogeneous morphologies
[28][61]. The impact of heating temperatures ranging from 20 °C to 90 °C, and brine concentrations ranging from 0.1% to 10%, on the optimal conditions for producing tofu, were explored. When the brine concentration was around 6%, the tofu had excellent morphologies, however, the texture of the tofu became tougher as the heating temperature increased. As a result, in subsequent studies, 6% brine and an 80 °C heating temperature was used.
Therapeutic cells can be delivered as living drugs by microcarriers, ideally in a spatiotemporally controlled manner. The ability to control the release of cells is important, because direct cell injection has been shown to result in greatly increased cell mortality, rendering the treatment ineffective
[29][30][62,63]. Another promising application of injectable cell-laden microcarriers, is their use in the development of tissue models for targeted drug delivery research
[31][32][64,65]. The use of advanced methods for delivering cells, to maximize the tissue repair potential, as well as to regenerate by stimulating angiogenic factors, has been demonstrated. Chara Simitzi et al. reported different surface topographies of hierarchically structured, porous biodegradable PLGA microcarriers, used for growing AdMSCs, and influence of microcarriers towards secretion of proangiogenic factors. Three different PLGA-based polymers, were used to fabricate microcarriers via thermally induced phase separation (TIPS)
[24][57]. Briefly, AdMSCs were grown on all three compositions of PLGA-TIPS microcarriers, under xeno-free conditions, for 11 days, LDH assay confirmed the cell viability of around 95%, and the results were compared with cells grown on tissue culture (TC) plates. The ability of trilineage differentiation has also been demonstrated for cells grown on PLGA-TIPS microcarriers. Multiple proangiogenic factors, including VEGF, were also amplified in the secretome of AdMSCs grown on microcarriers, indicating their ability to trigger angiogenesis.
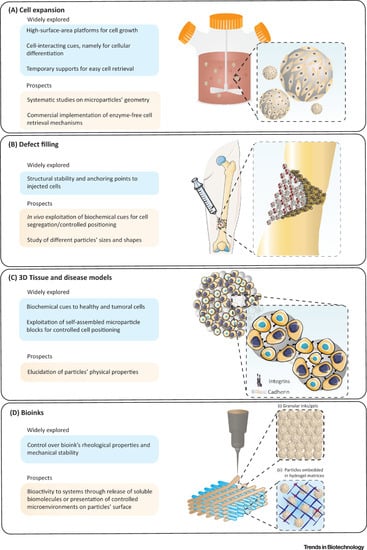
As per the
Figure 23 illustrations taken together, injectable biomaterials are promising candidates for fabricating a new class of biodegradable and injectable microcarriers that can generate and guide specific drug/cell responses, that match the biological environment towards defect filling, tissue repair and regeneration using 3D culture and bioprinting platforms
[33][34][66,67].
Figure 23. Schematic illustration to highlight: (
A) use of microcarrier in bioreactors for mass expansion and differentiation of cells. (
B) The microcarriers can be modified and injected into irregularly shaped defects, to effectively repair and enhance tissue recovery. (
C) Microcarriers in multicellular aggregates, as structural supports, to promote cell growth and differentiation in the 3D system. (
D) Advanced modular bioinks, that can accommodate (i) microcarriers tightly packed in the form of printable granular inks/gels; and (ii) microcarriers enabling the surrounding hydrogel matrix to mimic in vivo-like tissue architecture. Adapted with permission from
[25][58].