The polyadenylation (poly(A)) tail of mRNA is an essential feature that is required to mediate its nuclear export, stability, translation efficiency, and subcellular localization. Most genes have at least two mRNA isoforms via alternative splicing (AS) or alternative polyadenylation (APA), which increases the diversity of transcriptome and proteome.
- gene expression
- alternative polyadenylation
- stress response
- plants
1. Introduction
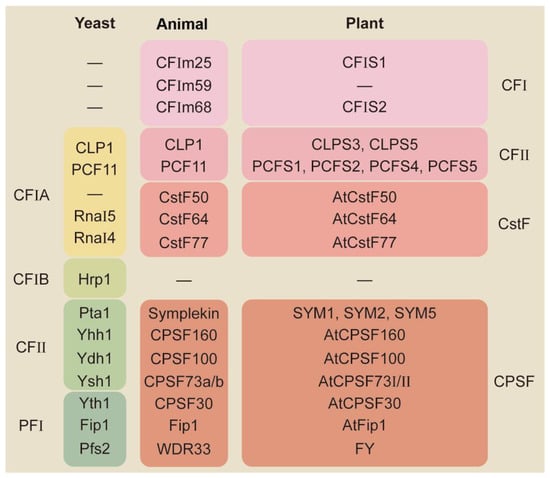
2. Molecular Mechanisms for APA-Mediated Responses
The mechanisms for the APA-mediated stress response have rarely been reported in plants; however, there are some reports from animals and yeast for the APA-mediated regulation of gene function.2.1. Influence Full-Length Transcripts
The poly(A) tail is added to the newly generated mRNA to indicate the termination of transcription and therefore determines the coding region of a protein. There are fewer studies on 5′UTR APA and CDS APA; more extensively studied is intronic APA, where polyadenylation occurring at the intron generally quickly encounters the termination codon to form a truncated protein, such that the truncated protein may be partially active or completely lose function [40][42]. Intronic APA events are common in human immune cells and severely dysregulated in cancer cells [41][42][43,44], suggesting an important role of intronic APA in the precise regulation of gene expression. Intronic APA events are often associated with competition between splicing and 3′-end-processing machinery on introns, even though eukaryote gene expression is generally considered to be co-transcribed [43][45]. There are interactions between splicing factor and polyadenylation factor [44][45][46,47], which may underlie the competition between them. Intronic APA events often occur in the first two introns, which often produce abnormally transcript-encoding short truncated protein and thus reduce the proportion of functional transcripts [46][48], or play a dominant negative role as the truncated protein inhibits the function of the full-length protein [47][49]. A certain percentage of introns are not fully spliced and a cryptic polyadenylation signal is recognized in the retained introns, which produces the truncated proteins under stress. Those retained introns are characterized by a length greater than the average length of introns, which may be close to the 5′UTR, implying that introns with weaker splicing signals may undergo intron retention under stress and be recognized by the 3′-end-formation machinery, which may act as competition for the function of full length and truncated proteins [48][49][50][50,51,52].2.2. Influence RNA Fate and Translation Efficiency
In contrast to intronic APA, APA occurring on the 3′UTR does not change the protein-coding sequence, but this does not mean that 3′UTR APA produces weaker effects than other forms of APA for the function of the target gene [51][53]. On the contrary, 3′UTR APA can alter key sequences that regulate RNA export and translation efficiency, and therefore, the regulation of 3′UTR length by APA is an important determinant for RNA fate determination. This mode of regulation is general, as more than 50% of the genes in the human genome show 3′UTR APA events [35].2.2.1. RNA Stability
Studies in yeast have shown that mRNA stability is negatively correlated to the length of 3′UTR, with transcripts with short half-lives being twice as long as the most stable transcripts [52][54]. The 3′UTR contains multiple cis-elements and is subject to complex regulation by trans-acting factors, such as microRNA or RNA-binding protein [53][55]. The more studied factors are the role of microRNA on the stability of mRNA with 3′UTR APA. MicroRNAs are short RNAs of about 20 nucleotides in size that regulate the post-transcriptional silencing of target genes [54][56]. The 3′UTR APA event often alters the sequence of the UTR in the mRNA to contain or not contain the microRNA-binding site. For example, Hsp70.3 promotes the use of the proximal site in the 3′UTR, which likewise increases the expression of Hsp70.3, thereby improving cell survival under heat shock conditions [55][57]. The shortening in NLRP3 3′UTR leads to the overactivation of NLRP3 and exacerbates the inflammatory response [56][58]. The up-regulation of the gene expression level for the genes with 3′UTR shortening is caused by avoiding microRNA-mediated degradation when shortening the 3′UTR. In addition to the microRNA, RNA-binding protein also acts via the 3′UTR as a post-transcriptional regulator. The binding of RNA-binding protein to mRNA may lead to the degradation of mRNA. The 3′UTR contains multiple cis-elements, ARE (Adenylate/uridylate Reich element) is the most well-known of these, being present in 5–8% of human genes and involved in regulating many important physiological processes [57][59]. Mouse cells use the proximal poly(A) site under arsenic stress and enhance the degradation of long 3′UTR transcription during recovery. The degradation of long 3′UTR transcription is due to the binding of TIA1 to the U-rich motif of the long 3′UTR, promoting SG recruitment and leading to enhanced mRNA decay [58][60]. Thus, the use of the proximal poly(A) site under stress facilitates the retention of transcript abundance.2.2.2. RNA Export
The transport of mRNA from the nucleus to the cytoplasm is a key process in the expression of genes in eukaryotes, and this process is closely controlled by the 3′UTR [59][61]. Biotic and abiotic stresses trigger the disruption of transcription termination (DoTT), which results in a read-through transcript that extends to the next gene instead of stopping at the 3′-end of the previous gene [60][62]. The read-through transcripts are strongly enriched in chromatin and soluble nuclear extracts and therefore cannot be efficiently exported to the cytoplasm. Thus, DoTT substantially regulates gene expression by inhibiting RNA export, which is associated with an overall decrease in the level of protein [61][63]. Under salt stress, 10% of human-encoded proteins produce transcriptional read-through events, and the poly(A) signal intensity of these genes is usually below the average, which may be the underlying cause of the read-through [62][64]. HSV-1 infection was followed by a significant transcriptional read-through of the interferon regulatory factor gene IRF1, which is important for the immune response against viruses, suggesting that transcriptional read-through may be one of the methods by which the virus escapes from host immunity [61][63].2.2.3. Translation Efficiency
Most mRNAs perform their functions at the protein level, and the 3′UTR can also regulate protein expression by affecting translation efficiency. In addition to participating in the regulation of RNA stability, microRNAs, and RNA-binding proteins, long 3′UTR also inhibits protein translation efficiency [63][65]. In general, the 3′UTR of proliferating cells is shorter than that of differentiating cells in higher animals [64][66]; correspondingly, mRNA isoforms with shorter 3′UTR exhibit higher translation efficiency [65][67]. Yeast grown on rich medium tended to express shorter transcripts compared to that on a mini medium, and shorter 3′UTR correlated with up-regulation of genes participating in translation [33]. Under oxidative stress, C/EBPγ, the target of mTOR signaling, appears shortened in 3′UTR; short transcripts have high translational efficiency, and high levels of C/EBPγ expression control redox homeostasis [66][68]. Therefore, 3′UTR APA can also rapidly regulate the expression of genes by regulating translation efficiency in subtle changes in the cellular and surrounding environment. In summary, the poly(A) site usage of many genes changes significantly under stress, such as the usage alteration of poly(A) site from the 3′UTR to the 5′UTR, introns and exons, and of course other positions in the 3′UTR. Genes usually escape from microRNA control due to the shortened 3′UTR and thus is up-regulated at the RNA level, which may facilitate the rapid response of resistance to stress, and 3′UTR shortening also improves translation efficiency and thus increases the expression at the protein level due to the presence of the binding motif in the 3′UTR for RNA-binding protein (Figure 2). The intronic APA and transcriptional read-through may down-regulate gene expression by reducing the ratio of functional transcripts or affecting the output of transcripts (Figure 2), and even transcriptional read-through may form long non-coding RNAs to regulate the expression of peripheral genes. At present, there are few studies on 5′UTR APA and exon APA, and they are also a strategy to reduce gene expression.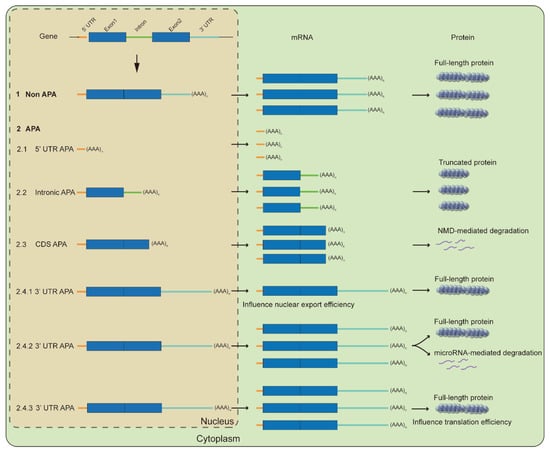
3. An Overview of the Role of APA in Response to Biotic and Abiotic Stresses
The regulation of gene expression is one of key steps for the development and response to environmental changes in eukaryotes. Plants require more precise regulation of gene expression than animals and yeast because they have smaller transcription units and intergenic regions [67][69]. Several recent reviews have summarized the role of APA on the regulation of plant growth and development [67][68][69][69,70,71]. Here, thwe researchers focus on the role of APA in the plant stress response. Stresses are commonly found in the environments where the organisms live, grow, and develop. Based on the characteristics of these stresses, they can be classified as biotic and abiotic stresses, which seriously affect the survival of plants and animals [70][72]. As sessile organisms, plants are more susceptible to stresses than animals. Throughout their life cycle, plants are constantly exposed to a variety of external stimuli. As a result, crop yields are affected to varying degrees by environmental stresses such as drought, salt, heavy metal, and hypoxic stresses. At the same time, plants also actively respond to environmental changes through the regulation of gene expression [71][73]. To overcome inevitable harsh environmental challenges, plants have evolved multiple gene-regulatory mechanisms to avoid injury. The studies indicate that a wide range of APA events occur under biotic and abiotic stresses in plants, suggesting that APA may act as a positive post-transcriptional regulation in response to stress in plants (Table 1). However, more detailed molecular mechanisms remain to be explored.|
Stress |
Species |
Target Genes |
APA Types |
Associated Polyadenylation Factors |
References |
|---|---|---|---|---|---|
|
Hypoxic |
Arabidopsis thaliana |
5′UTR APA CDS APA Intronic APA 3′UTR APA |
|||
|
Drought |
Arabidopsis thaliana |
3′UTR extension |
FPA |
||
|
Salt |
Arabidopsis thaliana Eutrema salsugineum Sorghum |
AKR2 AT3G47610 CIPK21 MAP3Kδ4 |
3′UTR APA |
FIP1 CPSF30 |
|
|
N starvation |
Arabidopsis thaliana Chlamydomonas |
NRT1.1 CPSF30 |
3′UTR APA Intronic APA |
FIP1 CPSF30 |
|
|
Temperature |
Arabidopsis thaliana |
SVK |
3′UTR extension |
||
|
Pathogens |
Rice Arabidopsis thaliana |
Xa1 rTGA2.1 CBP60g |
CDS APA 3′UTR APA |
FIP1 CPSF30 |
|
|
ROS |
Arabidopsis thaliana |
ERF4 |
CDS APA |
FPA CPSF30 |
