Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Mattia Bartoli.
Carbon quantum dots are the materials of a new era with astonishing properties such as high photoluminescence, chemical tuneability and high biocompatibility.
- carbon quantum dots
- nanomaterials
- optical properties
1. Introduction
Carbon materials have undergone several revolutions during the last century [1]. Several exciting discoveries have brought carbon science beyond the traditional use of graphite and carbon black. The rise of nanostructured carbon allotropes such as fullerene [2], carbon nanotubes (CNTs) [3] and graphene [4] has paved the way for a new era in material science. Curiously, fullerenes and CNTs were first observed by accident. This also happened for an entire new class of materials: carbon quantum dots (CDs). In 2004, Xu and co-workers [5] worked on the purification of oxidized CNTs using electrophoretic techniques and isolated a highly fluorescent fraction. This was the first description of CDs, even if a proper and clear identification had to wait until the work of Sun et al. [6]. Since then, CDs have gained great attention from the scientific community due to their high photoluminescence, solubility and size [7,8][7][8]. CDs have emerged as an easier to produce and handle alternative to inorganic quantum dots due to the fast and easy preparation routes [9] and several appealing features such as biocompatibility [10], solubility [11] and optical properties [12]. CDs have been tested for several kinds of advanced biological applications such as bioimaging [13[13][14],14], drug and gene delivery [15,16,17][15][16][17] and theranostics [18,19][18][19]. Furthermore, CDs can be used for other applications such as catalysts [20[20][21][22][23][24],21,22,23,24], sensing [25[25][26],26], environmental remediation [27,28][27][28] and filler for composite production [29].
The scientific community uses the term CDs to describe a quite heterogeneous set of nanoparticles with different chemical and optical features and only two common characteristics: nanometric size and high yield photoluminescence [30]. This loose definition is one of the issues related with carbon dots together while the relation between their properties and their chemical structure represents a major challenge in the field of a rational design of CDs [31]. These unfulfilled tasks hindered a proper critical approach to CD synthesis, and aimed to optimize their interactions with biological systems or to maximize their exploitation for targeting applications [32,33][32][33].
2. CDs’ Optical
Optical Properties
The most interesting and appealing characteristic of CDs is the great intensity of their fluorescence emission [62][34] that originates from quantum confinement (QC) occurring when the exciton’s Bohr radius is bigger than the average size of CDs [63][35]. The nanometer size of carbon dots does not allow the formation of crystal-like conduction, and the valence bands and the electronic level are discrete although somewhat broadened. The HOMO–LUMO gap increases when the CD size decreases, leading to the emission of photons in the UV region with an improvement in the quantum yield (QY) [64][36]. The fluorescence emission for CDs with π-domains of larger size (i.e., GQDs, CQNDs) is mainly due to conjugated π-electrons of the aromatic carbon domains [65][37]. Larger π-domains reduced the HOMO–LUMO gap and red shifted the fluorescence emission peak [62][34]. The same consideration holds for all xGQDs, although some adjustments are needed because of the presence of different heteroatoms. Considering CQNDs, Yu et al. [66][38] investigated another interesting effect on fluorescence coming from the core/shell size ratio and surface residues suggesting the fluorescence emission mechanism reported in Figure 1.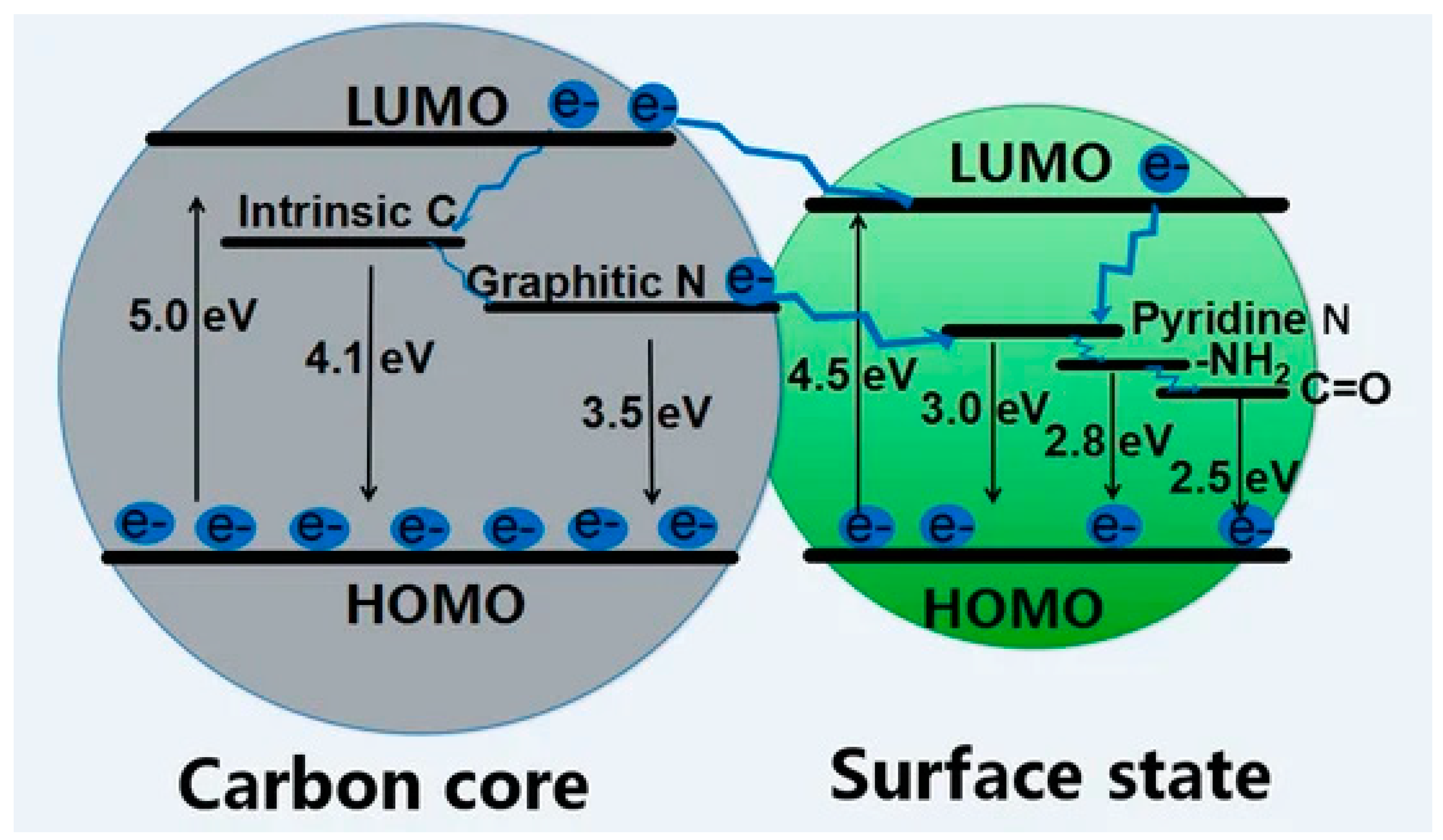
Figure 1. Scheme of the fluorescence emission of CQNDs based on different core/shell size ratios and surface residues.
References
- Ajayan, P.M. The nano-revolution spawned by carbon. Nature 2019, 575, 49–50.
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163.
- Radushkevich, L.; Lukyanovich, V.Á. O strukture ugleroda, obrazujucegosja pri termiceskom razlozenii okisi ugleroda na zeleznom kontakte. Zurn. Fisic. Chim. 1952, 26, 88–95.
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200.
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737.
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757.
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195.
- Zheng, C.; Tao, S.; Yang, B. Polymer–Structure-Induced Room-Temperature Phosphorescence of Carbon Dot Materials. Small Struct. 2023, 2200327.
- Sagbas, S.; Sahiner, N. Carbon dots: Preparation, properties, and application. In Nanocarbon and Its Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 651–676.
- Park, Y.; Kim, Y.; Chang, H.; Won, S.; Kim, H.; Kwon, W. Biocompatible nitrogen-doped carbon dots: Synthesis, characterization, and application. J. Mater. Chem. B 2020, 8, 8935–8951.
- Sanni, S.O.; Moundzounga, T.H.; Oseghe, E.O.; Haneklaus, N.H.; Viljoen, E.L.; Brink, H.G. One-Step Green Synthesis of Water-Soluble Fluorescent Carbon Dots and Its Application in the Detection of Cu2+. Nanomaterials 2022, 12, 958.
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381.
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939.
- Lin, L.; Rong, M.; Luo, F.; Chen, D.; Wang, Y.; Chen, X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. TrAC Trends Anal. Chem. 2014, 54, 83–102.
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon dots: An innovative tool for drug delivery in brain tumors. Int. J. Mol. Sci. 2021, 22, 11783.
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnol. 2020, 18, 142.
- Zhang, W.; Chen, J.; Gu, J.; Bartoli, M.; Domena, J.B.; Zhou, Y.; Ferreira, B.C.; Kirbas Cilingir, E.; McGee, C.M.; Sampson, R.; et al. Nano-carrier for gene delivery and bioimaging based on pentaetheylenehexamine modified carbon dots. J. Colloid Interface Sci. 2023, 639, 180–192.
- Ross, S.; Wu, R.-S.; Wei, S.-C.; Ross, G.M.; Chang, H.-T. The analytical and biomedical applications of carbon dots and their future theranostic potential: A review. J. Food Drug Anal. 2020, 28, 677.
- Mishra, V.; Patil, A.; Thakur, S.; Kesharwani, P. Carbon dots: Emerging theranostic nanoarchitectures. Drug Discov. Today 2018, 23, 1219–1232.
- Hutton, G.A.; Martindale, B.C.; Reisner, E. Carbon dots as photosensitisers for solar-driven catalysis. Chem. Soc. Rev. 2017, 46, 6111–6123.
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Carbon dot-based composites for catalytic applications. Green Chem. 2020, 22, 4034–4054.
- Yu, J.; Song, H.; Li, X.; Tang, L.; Tang, Z.; Yang, B.; Lu, S. Computational studies on carbon dots electrocatalysis: A review. Adv. Funct. Mater. 2021, 31, 2107196.
- Zhao, F.; Li, X.; Zuo, M.; Liang, Y.; Qin, P.; Wang, H.; Wu, Z.; Luo, L.; Liu, C.; Leng, L. Preparation of photocatalysts decorated by carbon quantum dots (CQDs) and their applications: A review. J. Environ. Chem. Eng. 2023, 11, 109487.
- Sendão, R.M.; Esteves da Silva, J.C.; Pinto da Silva, L. Applications of Fluorescent Carbon Dots as Photocatalysts: A Review. Catalysts 2023, 13, 179.
- Lo Bello, G.; Bartoli, M.; Giorcelli, M.; Rovere, M.; Tagliaferro, A. A Review on the Use of Biochar Derived Carbon Quantum Dots Production for Sensing Applications. Chemosensors 2022, 10, 117.
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180.
- Tran, N.-A.; Hien, N.T.; Hoang, N.M.; Dang, H.-L.T.; Van Quy, T.; Hanh, N.T.; Vu, N.H.; Dao, V.-D. Carbon dots in environmental treatment and protection applications. Desalination 2023, 548, 116285.
- Yang, X.; Sun, J.; Sheng, L.; Wang, Z.; Ye, Y.; Zheng, J.; Fan, M.; Zhang, Y.; Sun, X. Carbon dots cooperatively modulating photocatalytic performance and surface charge of O-doped g-C3N4 for efficient water disinfection. J. Colloid Interface Sci. 2023, 631, 25–34.
- Chen, J.; Xiao, G.; Duan, G.; Wu, Y.; Zhao, X.; Gong, X. Structural design of carbon dots/porous materials composites and their applications. Chem. Eng. J. 2021, 421, 127743.
- Jing, H.H.; Bardakci, F.; Akgöl, S.; Kusat, K.; Adnan, M.; Alam, M.J.; Gupta, R.; Sahreen, S.; Chen, Y.; Gopinath, S.C. Green Carbon Dots: Synthesis, Characterization, Properties and Biomedical Applications. J. Funct. Biomater. 2023, 14, 27.
- Banerjee, A.; Batabyal, S.K.; Pradhan, B.; Mohanta, K.; Bhattacharjee, R.R. Future perspectives of carbon quantum dots. In Carbon Quantum Dots for Sustainable Energy and Optoelectronics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 473–479.
- Zhang, R.; Hou, Y.; Sun, L.; Liu, X.; Zhao, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Li, R.; Wang, C. Recent advances in carbon dots: Synthesis and applications in bone tissue engineering. Nanoscale 2023, 15, 3106–3119.
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the methods for the synthesis of carbon dots and their emerging applications. Polymers 2021, 13, 3190.
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583.
- Connerade, J.P. A review of quantum confinement. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2009; pp. 1–33.
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381.
- Li, S.-Y.; He, L. Recent progresses of quantum confinement in graphene quantum dots. Front. Phys. 2022, 17, 33201.
- Yu, J.; Liu, C.; Yuan, K.; Lu, Z.; Cheng, Y.; Li, L.; Zhang, X.; Jin, P.; Meng, F.; Liu, H. Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions. Nanomaterials 2018, 8, 233.
- Sachdev, A.; Matai, I.; Gopinath, P. Implications of surface passivation on physicochemical and bioimaging properties of carbon dots. RSC Adv. 2014, 4, 20915–20921.
- Du, J.; Wang, H.; Wang, L.; Zhu, S.; Song, Y.; Yang, B.; Sun, H. Insight into the effect of functional groups on visible-fluorescence emissions of graphene quantum dots. J. Mater. Chem. C 2016, 4, 2235–2242.
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. Carbon 2013, 60, 421–428.
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25.
- Zhu, S.; Zhao, X.; Song, Y.; Lu, S.; Yang, B. Beyond bottom-up carbon nanodots: Citric-acid derived organic molecules. Nano Today 2016, 11, 128–132.
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294.
- Saberi, Z.; Rezaei, B.; Ensafi, A.A. Fluorometric label-free aptasensor for detection of the pesticide acetamiprid by using cationic carbon dots prepared with cetrimonium bromide. Microchim. Acta 2019, 186, 273.
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. 2015, 127, 5450–5453.
- Pan, L.; Sun, S.; Zhang, A.; Jiang, K.; Zhang, L.; Dong, C.; Huang, Q.; Wu, A.; Lin, H. Truly Fluorescent Excitation-Dependent Carbon Dots and Their Applications in Multicolor Cellular Imaging and Multidimensional Sensing. Adv. Mater. (Deerfield Beach Fla.) 2015, 27, 7782–7787.
- Tao, S.; Song, Y.; Zhu, S.; Shao, J.; Yang, B. A new type of polymer carbon dots with high quantum yield: From synthesis to investigation on fluorescence mechanism. Polymer 2017, 116, 472–478.
- Zhu, S.; Song, Y.; Shao, J.; Zhao, X.; Yang, B. Non-conjugated polymer dots with crosslink-enhanced emission in the absence of fluorophore units. Angew. Chem. Int. Ed. 2015, 54, 14626–14637.
- Liu, S.G.; Luo, D.; Li, N.; Zhang, W.; Lei, J.L.; Li, N.B.; Luo, H.Q. Water-soluble nonconjugated polymer nanoparticles with strong fluorescence emission for selective and sensitive detection of nitro-explosive picric acid in aqueous medium. ACS Appl. Mater. Interfaces 2016, 8, 21700–21709.
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914.
- Hu, C.; Lin, T.-J.; Huang, Y.-C.; Chen, Y.-Y.; Wang, K.-H.; Lin, K.-Y.A. Photoluminescence quenching of thermally treated waste-derived carbon dots for selective metal ion sensing. Environ. Res. 2021, 197, 111008.
- Xiong, Y.; Schneider, J.; Ushakova, E.V.; Rogach, A.L. Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 2018, 23, 124–139.
- Lee, H.; Su, Y.-C.; Tang, H.-H.; Lee, Y.-S.; Lee, J.-Y.; Hu, C.-C.; Chiu, T.-C. One-pot hydrothermal synthesis of carbon dots as fluorescent probes for the determination of mercuric and hypochlorite ions. Nanomaterials 2021, 11, 1831.
- Rajendran, S.; Ramanaiah, D.V.; Kundu, S.; Bhunia, S.K. Yellow Fluorescent Carbon Dots for Selective Recognition of As3+ and Fe3+ Ions. ACS Appl. Nano Mater. 2021, 4, 10931–10942.
- Wang, L.; Jana, J.; Chung, J.S.; Hur, S.H. Glutathione modified N-doped carbon dots for sensitive and selective dopamine detection. Dye. Pigment. 2021, 186, 109028.
- Fu, Y.; Huang, L.; Zhao, S.; Xing, X.; Lan, M.; Song, X. A carbon dot-based fluorometric probe for oxytetracycline detection utilizing a Förster resonance energy transfer mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118947.
- Zhang, H.; Liu, J.; Wang, B.; Liu, K.; Chen, G.; Yu, X.; Li, J.; Yu, J. Zeolite-confined carbon dots: Tuning thermally activated delayed fluorescence emission via energy transfer. Mater. Chem. Front. 2020, 4, 1404–1410.
- Li, G.; Fu, H.; Chen, X.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots based on inner filter effect. Anal. Chem. 2016, 88, 2720–2726.
- Liu, S.; Zhao, N.; Cheng, Z.; Liu, H. Amino-functionalized green fluorescent carbon dots as surface energy transfer biosensors for hyaluronidase. Nanoscale 2015, 7, 6836–6842.
- Sharma, S.; Umar, A.; Sood, S.; Mehta, S.C.; Kansal, S.K. Photoluminescent C-dots: An overview on the recent development in the synthesis, physiochemical properties and potential applications. J. Alloys Compd. 2018, 748, 818–853.
- Gonçalves, H.; Esteves da Silva, J.C.G. Fluorescent Carbon Dots Capped with PEG200 and Mercaptosuccinic Acid. J. Fluoresc. 2010, 20, 1023–1028.
- Wang, F.; Hao, Q.; Zhang, Y.; Xu, Y.; Lei, W. Fluorescence quenchometric method for determination of ferric ion using boron-doped carbon dots. Microchim. Acta 2016, 183, 273–279.
- Fraiji, L.K.; Hayes, D.M.; Werner, T. Static and dynamic fluorescence quenching experiments for the physical chemistry laboratory. J. Chem. Educ. 1992, 69, 424.
- Xu, J.; Sahu, S.; Cao, L.; Bunker, C.E.; Peng, G.; Liu, Y.; Fernando, K.A.S.; Wang, P.; Guliants, E.A.; Meziani, M.J.; et al. Efficient Fluorescence Quenching in Carbon Dots by Surface-Doped Metals—Disruption of Excited State Redox Processes and Mechanistic Implications. Langmuir 2012, 28, 16141–16147.
- Gong, J.; Lu, X.; An, X. Carbon dots as fluorescent off–on nanosensors for ascorbic acid detection. RSC Adv. 2015, 5, 8533–8536.
- Wang, S.; Deng, G.; Yang, J.; Chen, H.; Long, W.; She, Y.; Fu, H. Carbon dot- and gold nanocluster-based three-channel fluorescence array sensor: Visual detection of multiple metal ions in complex samples. Sens. Actuators B Chem. 2022, 369, 132194.
- Song, Y.; Zhu, S.; Xiang, S.; Zhao, X.; Zhang, J.; Zhang, H.; Fu, Y.; Yang, B. Investigation into the fluorescence quenching behaviors and applications of carbon dots. Nanoscale 2014, 6, 4676–4682.
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence Quenching by Photoinduced Electron Transfer: A Reporter for Conformational Dynamics of Macromolecules. ChemPhysChem 2009, 10, 1389–1398.
- Sahoo, H. Förster resonance energy transfer—A spectroscopic nanoruler: Principle and applications. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 20–30.
- Wang, X.; Cao, L.; Lu, F.; Meziani, M.J.; Li, H.; Qi, G.; Zhou, B.; Harruff, B.A.; Kermarrec, F.; Sun, Y.-P. Photoinduced electron transfers with carbon dots. Chem. Commun. 2009, 25, 3774–3776.
- Narayanan, R.; Deepa, M.; Srivastava, A.K. Förster resonance energy transfer and carbon dots enhance light harvesting in a solid-state quantum dot solar cell. J. Mater. Chem. A 2013, 1, 3907–3918.
- Miao, S.; Liang, K.; Kong, B. Förster resonance energy transfer (FRET) paired carbon dot-based complex nanoprobes: Versatile platforms for sensing and imaging applications. Mater. Chem. Front. 2020, 4, 128–139.
- Yoo, H.J.; Kwak, B.E.; Kim, D.H. Competition of the roles of π-conjugated domain between emission center and quenching origin in the photoluminescence of carbon dots depending on the interparticle separation. Carbon 2021, 183, 560–570.
- Guillet, J. Polymer Photophysics and Photochemistry; John Wiley & Sons: New York, NY, USA, 1985.
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26.
- Vaishnav, J.K.; Mukherjee, T.K. Long-range resonance coupling-induced surface energy transfer from CdTe quantum dot to plasmonic nanoparticle. J. Phys. Chem. C 2018, 122, 28324–28336.
- Amjadi, M.; Abolghasemi-Fakhri, Z.; Hallaj, T. Carbon dots-silver nanoparticles fluorescence resonance energy transfer system as a novel turn-on fluorescent probe for selective determination of cysteine. J. Photochem. Photobiol. A Chem. 2015, 309, 8–14.
- Liu, X.; Xu, Z.; Cole, J.M. Molecular Design of UV–vis Absorption and Emission Properties in Organic Fluorophores: Toward Larger Bathochromic Shifts, Enhanced Molar Extinction Coefficients, and Greater Stokes Shifts. J. Phys. Chem. C 2013, 117, 16584–16595.
- Araneda, J.F.; Piers, W.E.; Heyne, B.; Parvez, M.; McDonald, R. High Stokes Shift Anilido-Pyridine Boron Difluoride Dyes. Angew. Chem. Int. Ed. 2011, 50, 12214–12217.
- Krishnaiah, P.; Atchudan, R.; Perumal, S.; Salama, E.-S.; Lee, Y.R.; Jeon, B.-H. Utilization of waste biomass of Poa pratensis for green synthesis of n-doped carbon dots and its application in detection of Mn2+ and Fe3+. Chemosphere 2022, 286, 131764.
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447.
- Del Valle, J.C.; Catalán, J. Kasha’s rule: A reappraisal. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069.
- Khan, S.; Gupta, A.; Verma, N.C.; Nandi, C.K. Time-Resolved Emission Reveals Ensemble of Emissive States as the Origin of Multicolor Fluorescence in Carbon Dots. Nano Lett. 2015, 15, 8300–8305.
- Demchenko, A.P.; Dekaliuk, M.O. The origin of emissive states of carbon nanoparticles derived from ensemble-averaged and single-molecular studies. Nanoscale 2016, 8, 14057–14069.
- Thiyagarajan, S.K.; Raghupathy, S.; Palanivel, D.; Raji, K.; Ramamurthy, P. Fluorescent carbon nano dots from lignite: Unveiling the impeccable evidence for quantum confinement. Phys. Chem. Chem. Phys. 2016, 18, 12065–12073.
- Li, W.; Zhou, W.; Zhou, Z.; Zhang, H.; Zhang, X.; Zhuang, J.; Liu, Y.; Lei, B.; Hu, C. A universal strategy for activating the multicolor room-temperature afterglow of carbon dots in a boric acid matrix. Angew. Chem. 2019, 131, 7356–7361.
- Tao, S.; Lu, S.; Geng, Y.; Zhu, S.; Redfern, S.A.T.; Song, Y.; Feng, T.; Xu, W.; Yang, B. Design of Metal-Free Polymer Carbon Dots: A New Class of Room-Temperature Phosphorescent Materials. Angew. Chem. Int. Ed. 2018, 57, 2393–2398.
- Song, S.-Y.; Liu, K.-K.; Cao, Q.; Mao, X.; Zhao, W.-B.; Wang, Y.; Liang, Y.-C.; Zang, J.-H.; Lou, Q.; Dong, L.; et al. Ultraviolet phosphorescent carbon nanodots. Light Sci. Appl. 2022, 11, 146.
- Liang, Y.-C.; Gou, S.-S.; Liu, K.-K.; Wu, W.-J.; Guo, C.-Z.; Lu, S.-Y.; Zang, J.-H.; Wu, X.-Y.; Lou, Q.; Dong, L.; et al. Ultralong and efficient phosphorescence from silica confined carbon nanodots in aqueous solution. Nano Today 2020, 34, 100900.
- Jia, J.; Lu, W.; Gao, Y.; Li, L.; Dong, C.; Shuang, S. Recent advances in synthesis and applications of room temperature phosphorescence carbon dots. Talanta 2021, 231, 122350.
- Knoblauch, R.; Bui, B.; Raza, A.; Geddes, C.D. Heavy carbon nanodots: A new phosphorescent carbon nanostructure. Phys. Chem. Chem. Phys. 2018, 20, 15518–15527.
- Wei, X.; Yang, J.; Hu, L.; Cao, Y.; Lai, J.; Cao, F.; Gu, J.; Cao, X. Recent advances in room temperature phosphorescent carbon dots: Preparation, mechanism, and applications. J. Mater. Chem. C 2021, 9, 4425–4443.
- Hu, G.; Xie, Y.; Xu, X.; Lei, B.; Zhuang, J.; Zhang, X.; Zhang, H.; Hu, C.; Ma, W.; Liu, Y. Room temperature phosphorescence from Si-doped-CD-based composite materials with long lifetimes and high stability. Opt. Express 2020, 28, 19550–19561.
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2019, 196, 456–478.
- Lin, Z.; Xue, W.; Chen, H.; Lin, J.-M. Classical oxidant induced chemiluminescence of fluorescent carbon dots. Chem. Commun. 2012, 48, 1051–1053.
- Shen, C.-L.; Lou, Q.; Liu, K.-K.; Dong, L.; Shan, C.-X. Chemiluminescent carbon dots: Synthesis, properties, and applications. Nano Today 2020, 35, 100954.
- Xu, Y.; Wu, M.; Feng, X.Z.; Yin, X.B.; He, X.W.; Zhang, Y.K. Reduced carbon dots versus oxidized carbon dots: Photo-and electrochemiluminescence investigations for selected applications. Chem.—Eur. J. 2013, 19, 6282–6288.
More
