Cancer represents one of the most pernicious public health problems with a high mortality rate among patients worldwide. Chemotherapy is one of the major therapeutic approaches for the treatment of various malignancies. Platinum-based drugs (cisplatin, oxaliplatin, carboplatin, etc.) are highly effective chemotherapeutic drugs used for the treatment of several types of malignancies, but their application and dosage are limited by their toxic effects on various systems, including neurotoxicity. Simultaneously, researchers have tried to improve the survival rate and quality of life of cancer patients and decrease the toxicity of platinum-containing drugs by combining them with non-chemotherapy-based drugs, dietary supplements and/or antioxidants. Additionally, recent studies have shown that the root cause for the many side effects of platinum chemotherapeutics involves the production of reactive oxygen species (ROS) in naive cells. Therefore, suppression of ROS generation and their inactivation with antioxidants represents an appropriate approach for platinum drug-induced toxicities. The aim of this paper is to present an updated review of the protective effects of different antioxidant agents (vitamins, dietary antioxidants and supplements, medicaments, medicinal plants and their bioactive compounds) against the neurotoxicity induced by platinum-based chemotherapeutics. This review highlights the high potential of plant antioxidants as adjuvant strategies in chemotherapy with platinum drugs.
- platinum-based drugs
- cisplatin
- carboplatin
- oxaliplatin
- neurotoxicity
- peripheral neuropathy
- antioxidants
1. Introduction
A “honeymoon” with platinum-based compounds in chemotherapy that officially started by pronouncing cisplatin as “the drug of the century” more than 50 years ago has gradually been overshadowed by very serious therapeutic limitations of those drugs. Besides the inherited resistance to treatment with any of the currently approved platinum agents, each of them has a number of clinically confirmed side-effects, ranging from minor to dose-limiting. The fact that almost half of all the patients who receive anticancer chemotherapy are treated with a platinum drug[1] [1] gives a good insight into broad-spectrum platinum-based chemotherapeutics adverse effects.
2. Therapeutic Indications for Platinum-Based Chemotherapy
For decades various platinum-based compounds were employed as an important part of the combination chemotherapy regimens used to treat different types of solid tumors. However, there are only three platinum-based medications that are used throughout the world for the cancer treatment: cisplatin (cis-diamminedichloridoplatinum II), carboplatin (cis-diammine-1,1-cyclobutanedicarboxylateplatinum), and oxaliplatin (trans-R,R-cyclohexane-1,2-diamineoxalato- platinum II), while some other platinum-based therapeutics are approved only in individual countries, such as heptaplatin (Korea), lobaplatin (China), miriplatin (Japan), and nedaplatin (Japan) [2][2].
Since 1979 cisplatin has been widely used (along with other antineoplastic drugs) in the treatment of various malignancies: lung [3][3], ovarian [4][4], testicular [5][5], breast[6] [6] and brain cancer [7][7], sarcomas [8][8], and lymphomas [9][9]. Starting in 1989, carboplatin confirmed clinical relevance as an antineoplastic agent (in combination with other chemotherapeutics) for advanced ovarian carcinoma [10][10], head and neck cancer [11][11], and lung cancer [12][12]. The latest worldwide accepted platinum-based chemotherapeutic (2002), oxaliplatin, is used as a part of the therapeutic protocols for metastatic colorectal cancer [13][13], advanced gastric[14] [14] and ovarian cancer[15]. [15].
3. Side Effects of Platinum-Based Compounds in Clinical Practice
Like for the other chemotherapeutic drugs, the basic cytotoxic effect of platinum-based compounds (DNA damage) is not restricted only to target tissue (tumor cells), but is also affecting numerous organ systems in patients receiving chemotherapy, resulting in a variety of side effects. Based on a similar pathophysiological background, the adverse effects of platinum-based chemotherapeutics may be classified in certain categories of toxicities according to their clinical manifestations. The most commonly described types of side effects associated with platinum-based treatment are usually classified as nephrotoxicity, hepatotoxicity, neuro- and ototoxicity, cardiotoxicity, hematological toxicity, and gastrointestinal toxicity. However, patients’ responses to chemotherapy toxicity, including adverse effects of platinum-based compounds, are significantly determined by several factors, such as age, gender, drug administration schedule and performance status [16][16].
Nausea and vomiting are considered as the most common clinical manifestations of side effects following cisplatin administration. Strongly depending on the applied dose, this effect of cisplatin, which can be successfully abolished by antiemetic action of 5-HT3 antagonists [16][16], is found more often than in chemotherapeutic protocols with oxaliplatin and carboplatin [17][17].
Nephrotoxicity represents the main limitation for chemotherapeutic protocols that involved platinum-based compounds which is not surprising due to the fact that kidneys are the main route for platinum compounds excretion. Although the platinum-based drugs affect all three key kidney functions (filtration, reabsorption, and excretion), the two most common nephrotoxic side effects of cisplatin are acute kidney injury (also known as acute renal failure) and hypomagnesemia, which is reported to affect up to 90% of cisplatin-treated patients [2][2]. However, the comparison of toxicities for three platinum-containing chemotherapy regimens confirms the lower nephrotoxicity manifestations of both carbo- and oxaliplatin when compared to cisplatin [18][18].
Hepatotoxicity is also considered as one of the most frequent adverse effects of platinum-based compounds administration in clinical practice. Morphological alterations accompanying platinum-based compounds application are manifested as necrosis, degeneration of hepatocytes, and increased inflammatory response [19][19], as well as a consequent increase in hepatic enzymes and bilirubin [20][20]. Long-term survival analysis of platinum-based compounds-induced hepatotoxicity showed that liver damage was more pronounced following cisplatin administration when compared to carboplatin [21][21], while drug-induced hepatotoxicity manifestations accompanying oxaliplatin therapy were predominantly restricted to hepatic vascular injury[22]. [22].
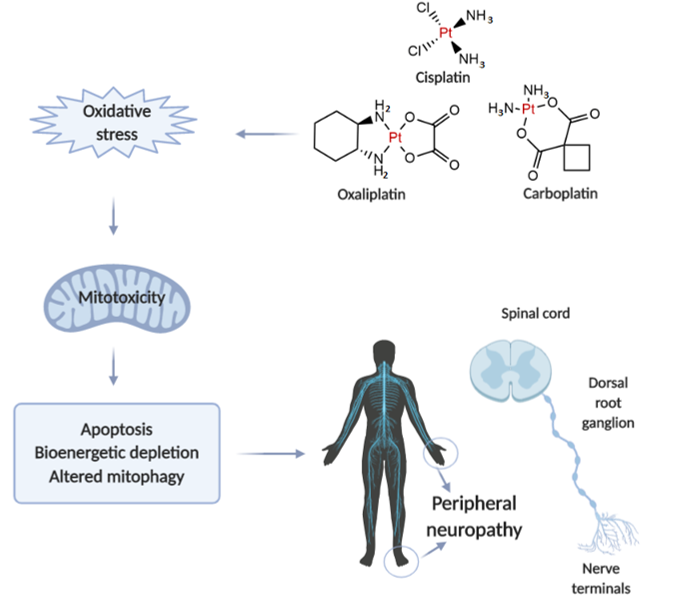
Neurotoxicity in response to platinum-based therapy is the leading clinical entity, aside from nephro- and hepatotoxicity that usually hampers platinum-based chemotherapy (Figure 1). The most frequently reported manifestations of neurotoxicity are due to the clinical appearance of peripheral neurotoxicity (numbness, tingling, or paresthesia in fingers and/or toes). With prolonged treatment, they gradually lead to disturbance of proprioception, which may result in ataxic gait [23][23]. The clinical manifestations of encephalopathy accompanying platinum-based therapy usually appear with an increase in cumulative dose [24][24]. Sensory manifestations of platinum compounds-induced neuropathy are often accompanied by ototoxicity[25]. [25].
When comparing to the individual extension of neurotoxic manifestations for platinum-based drugs, it has been shown that carboplatin neurotoxicity is negligible compared to cisplatin and oxaliplatin. However, carboplatin, particularly when applied in high doses, can lead to the development of neurotoxic manifestations that may become irreversible in 30-50% of patients.
Figure 1. Illustrative presentation of the development of platinum-based drug-induced peripheral neuropathy. Created in BioRender.com.
The evaluation of mechanisms underlying platinum-induced peripheral neurotoxicity (PIPN), in both in vivo and in vitro studies, confirmed that those compounds after passing the neuronal membrane initiate several proapoptotic phenomena including the activation of p53, Bax translocation, cytochrome c release, as well as the activation of caspase-3 and 9 [26][26]. Similar to the beneficial action of platinum compounds on tumor’s nuclear DNA, the undesirable impact has been observed on naïve cells mitochondrial DNA which results in inhibition of replication and translation, with consequent respiratory chain disturbance and energy deficiency [27][27]. Finally, the platinum compounds-induced mitochondrial dysfunction resulted in increased reactive oxygen species (ROS) production (with oxidative damage) and intracellular calcium accumulation [27][27]. Furthermore, the observed intracellular calcium accumulation was potentiated via up-regulation of calcium N channels induced by platinum compounds, that additionally potentiated apoptotic mechanisms [28][28]. An additional transmembrane mechanism is involved in the neurotoxic effect of platinum compounds on peripheral nerves. Namely, it has been reported that cisplatin-induced neurotoxicity increased expression of TRPA1 receptors in dorsal root ganglia that resulted in hyperalgesic response to thermal stimuli[29]. [29].
Interestingly, although it is still very questionable how cisplatin passes through an intact blood-brain barrier, there are certain literature data considering the central manifestations of neurotoxicity induced by platinum compounds. It has been shown that cisplatin administration strongly affects CNS progenitor cells and oligodendrocytes inducing the apoptotic events in hippocampal dentate gyrus and corpus callosum [30][30]. Only a few studies implicate that several mechanisms of platinum compounds action in CNS, including oxidative damage, inflammation and apoptosis, may be the cause of behavioral alterations manifested as increased anxiety [31][31], depression [32][32], as well as cognitive dysfunction [33][33].
Since there is a plethora of evidence that oxidative damage is crucially involved in the mechanisms of platinum-based compounds toxicities, it is not surprising that an enormous effort has been put in order to increase the safety of platinum-based therapy regimens by promoting the antioxidant supplementation as potentially protective interventions during the chemotherapy protocols-based platinum-containing antitumor agents.
34. Antioxidants in the Treatment of Platinum-Based Chemotherapeutics-Induced Neurotoxicity
3.1. Vitamins, Minerals, and Dietetic Supplements
4.1. Vitamins, Minerals, and Dietetic Supplements
According to the World Health Organization recommendations nutrition is one of the major modifiable determinants of chronic disease [46][34]. Some essential nutrients, micronutrients, minerals, and trace elements, as well as nutritional supplements, beside their primary role for adequate functioning of an organism, possess anti-inflammatory, antihyperalgesic, and antioxidant effects through which they can influence on a chronic disease. One of the preventive and therapeutic strategies for alleviating neurotoxicity side effects in patients receiving platinum-based chemotherapeutics is the use of supplementation including vitamins, minerals and trace elements, as well as dietary supplements with antioxidant activity.
34.2. Clinically Used Medications
Amifostine is an organic thiophosphate that has cytoprotective and detoxicant activities. It is generally an inactive prodrug which activates after dephosphorilation in plasma membrane with alkaline phosphatase. When active metabolite enters the cell it act like free radical scavenger protecting DNA and cellular membranes [98][35]. There are many in vitro reports that showed good neuroprotective properties of amifostine against cisplatin and oxaliplatin, and some studies on patients claiming promising effects of amifostine against peripheral neurotoxicity induced by cisplatin, oxaliplatin, and carboplatin [99,100][36][37]. Moreover, a network meta-analysis of Fu and coworkers[38] [76] confirmed that amifostine was the most active against both overall and severe platinum drugs-induced neurotoxicities compared to other most used therapies, such as vitamin E, GSH, and Ca/Mg infusion. Metformin, an anti-diabetic drug, showed neuroprotective effects against other chemotherapy-induced neuropathies, and therefore it has recently been tested in vivo for alleviating the oxaliplatin-induced neuropathy in rats. It was able to decrease levels of ROS and markers of oxidative stress and to ameliorate intraepidermal fibers degeneration, gliosis, and sensitivity [77][39]. The new drug in the treatment of multiple sclerosis - dimethyl fumarate (DMF) was tested because of its neuroprotective properties on cisplatin and oxaliplatin-induced neurodegeneration in the in vitro study [78][40]. DMF induced up-regulation of the nuclear factor-erythroid-2-related factor 2 (Nrf2)-dependent antioxidant response and prevented the inhibition of neurite outgrowth. The antioxidant and mitoprotective potential of carvedilol, an antihypertensive drug, was tested in vitro on neuronal cells (Neuro-2a) affected by oxaliplatin. Carvedilol is a non-selective beta-adrenergic receptor blocker (β1,β2) of the third generation, and alpha adrenergic receptor blocker (α1) exerting antioxidant potential. It showed significant antioxidant and free radical scavenging effects with the alleviation of functional and sensorimotor deficits, but without in vitro affecting the anti-tumor effects of oxaliplatin [79][41].
Cisplatin-induced neurotoxicity in rats was treated with oxytocin, a neurohypophyseal nonapeptide synthesized in the hypothalamus [80][42]. The results showed that oxytocin was able to reduce oxidative stress and inflammation in rats, but also to mitigate the electromyography (EMG) changes in rats treated with cisplatin. Another compound that can reduce the oxidative stress induced by oxaliplatin in rats is phosphatidylcholine [81], but also exhibited potential in the regulation of microglial activation and thus decreasing peripheral neuropathy in rats. In the study of Chiu et al.[43] [34] chemotherapy (cisplatin)-induced cognitive impairment was treated with pifithrin-μ, an inhibitor of mitochondrial p53 accumulation. The use of this small molecule led to a significant improvement in preserving neuronal mitochondrial function. Patients with colorectal cancer treated with oxaliplatin-based chemotherapy were receiving co-treatment with monosialotetrahexosyl- ganglioside, known for its impacts on neuronal plasticity and repair mechanisms, and the release of neurotrophins in the brain [82][44]. This medication was able to significantly reduce the incidence of neuropathy induced by oxaliplatin, particularly severe neuropathy, with the absence of interfering with oxaliplatin activity.
34.3. Natural Products and Medicinal Plants
Compounds of the natural origin are in use in traditional and modern medicine regarding their numerous beneficial effects. They can be used in the prevention and/or therapy of various pathological conditions such as cardiovascular diseases, metabolic disorders, carcinogenesis, and neural impairments [105].
Compounds of the natural origin are in use in traditional and modern medicine regarding their numerous beneficial effects. They can be used in the prevention and/or therapy of various pathological conditions such as cardiovascular diseases, metabolic disorders, carcinogenesis, and neural impairments[45].
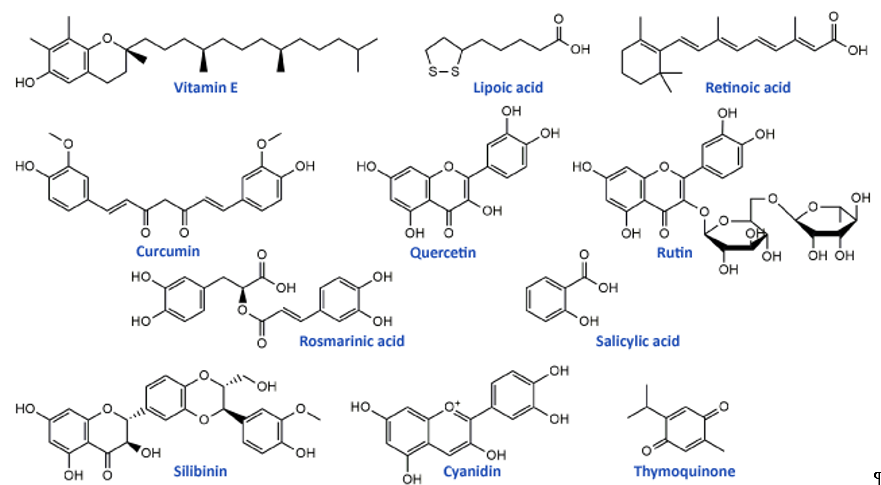
Flavonoids, the most common class of polyphenolic compounds in the plant kingdom, are well-known for their biological potential which mostly lies in the fact that they have exceptional antioxidant properties [140][46]. Quercetin, a bioactive flavonol, and its glucoside rutin (quercetin-3-O-rutinoside) (Figure 2) were tested for their ability to restore increased thermal and mechanical nociceptive response induced by oxaliplatin in mice [114][47]. Both compounds significantly reduced oxidative stress and prevented oxaliplatin-induced chronic painful peripheral neuropathy. Rutin confirmed its neuroprotective action against cisplatin in an in vivo study conducted by Almutairi and coworkers [115][48]. The high expressions of PON-1, PON-3, PPAR-δ, and GPX in rat brain tissues caused by cisplatin were restored by rutin application while PON-2 expression levels were increased. It was clear that rutin manifests its activity via the antioxidant pathway. Another flavonoid compound, 6-methoxyflavon, was tested against cisplatin-induced neuropathic allodynia and hypoalgesia in rats [116][49]. This compound exerted both peripheral and central antinociceptive activities, reducing the chemotherapy-induced peripheral neuropathy, but with the absence of motor side-effects characteristic to gabapentin as a control.
Phenolic acids are known for their significant antioxidant, antitumor, and antimicrobial activity and thanks to that they display many benefits on human health [141,142][50][51]. Many members of this group of phenolics showed powerful effects against different neurological disorders acting as neuroprotectors [143][52]. These compounds also showed significant alleviation of disrupted parameters during the cisplatin treatment, particularly rosmarinic acid [144][53], ellagic acid [145][54], protocatechuic acid [146][55], and caffeic acid phenethyl ester [147][56]. Therefore, it is not a surprising fact that rosmarinic acid (Figure 2) was able to mitigate mitochondrial dysfunction and spinal glial activation in vitro and in vivo in oxaliplatin-induced peripheral neuropathy [119][57]. Salicylic acid (Figure 2) showed a reduction in cisplatin neurotoxicity by antioxidant effects in rat primary neuron cell cultures in vitro [117][58]. Moreover, in cisplatin-induced neurotoxicity in rats, caffeic acid phenethyl ester, a derivative of caffeic acid, was capable to restore to normal activities of brain metabolic enzymes (hexokinase, glucose-6-phosphate dehydrogenase, lactate dehydrogenase, and malate dehydrogenase) showing vital activity against the development of neuropathy[59]. [118].
In the study of Li et al.[57] [120] cyanidin, a natural phenolic compound belonging to the group of anthocyanidins present in many fruits and vegetables, especially colored berries, cherries and grapes (Figure 2), was able to suppress oxidative stress in cisplatin-induced neurotoxicity on PC12 cells based on its notable antioxidant potential against ROS overproduction. The main bioactive compound of Nigela sativa (black cumin) seed oil is thymoquinone (Figure 2). Due to its antioxidant, anti-inflammatory, anti-neoplastic, and neuroprotective properties, it exhibited protective activity against cisplatin neurotoxicity in cultured DRG neurons [121][60]. Namely, thymoquinone promoted the neuronal cell viability and neurite outgrowth in a dose-dependent manner. In another in vivo study, thymoquinone reduced oxidative stress downregulated the apoptotic markers (p38 mitogen-activated protein kinase (MAPK), STAT-1, p53, p21, and MMP9) in rats and protected brain tissue against cisplatin action [122][61]. In the same study [122][61], geraniol, monoterpenoid alcohol, showed similar effects as thymoquinone during cisplatin-induced neurotoxicity in rats. Chen et al.[62] [123] studied the effects of ginsenoside Rb1, a ginsenoside found in Panax ginseng and Panax japonicus var. major roots, on cisplatin-induced memory impairments in rats. In assays such as novel objects recognition task and Morris water maze task it was shown that ginsenoside Rb1 significantly ameliorated memory impairments caused by cisplatin, as well as reduced the neuronal loss induced by cisplatin in different regions (CA1, CA3, and dentate gyrus) of the hippocampus. Moreover, this compound exhibited the ability to rescue the cholinergic neuron function in rat brain and also lowered oxidative stress and neuroinflammation.
Isothiocyanates, a group of natural bioactive compounds mostly present in cruciferous vegetables, are characterized by their antioxidant properties [148][63]. That is what enabled them to be used in the alleviation of platinum drug-induced neurotoxicity. Allyl-isothiocyanate alleviated neuropathic pain induced by oxaliplatin in rats and reduced the hypersensitivity to cold non-noxious stimuli by releasing H2S [124][64]. Similarly, glucosinolate glucoraphanin and derived isothiocyanate sulforaphane exerted reducing effects on oxaliplatin induced-neuropathic pain in mice, in a dose-dependent manner by releasing H2S and modulating Kv7 channels [125][65]. The same research group, Di Cesare Mannelli et al.[66] [126] conducted in vivo experiments on oxaliplatin-induced neuropathy using silibinin as an antioxidant compound. Silibinin (Figure 2) is a flavonolignan isolated from Silybum marianum that possesses antioxidant and antineoplastic activities. In this study, silibinin reduced oxidative damage of oxaliplatin in rats at a concentration of 100 mg/kg. It also recovered motor coordination and showed a reduction in oxaliplatin-dependent pain induced by mechanical and thermal stimuli. Silibinin is one of the components in silymarin complex mixture which also contains three more flavonolignans (silychristin, silydianin, and taxifolin) and its use is mainly focused on liver disorders treatment [149][67]. Silymarin in rats, at a concentration of 100 mg/kg, decreased the anxiogenic effect of cisplatin treatment and exhibited significant antioxidant activity in brain tissues by lowering lipid peroxidation and elevating GSH levels and CAT and SOD activities[68]. [127].
Figure 2. Chemical structures of some supplements and natural antioxidants (from Tables 2 and 3) that exhibited neuroprotective potential against platinum drugs-induced neurotoxicities.
Many herbal mixtures are in use for relieving chemotherapy-induced neuropathic pain. One that showed significant improvement regarding the negative effects of platinum-based drugs is goshajinkigan, a traditional Japanese Kampo medicine consisted of ten medicinal plants that is used clinically to treat pain in Japan. Ushio et al.[69] [128] showed that goshajinkigan was able to prevent cold hyperalgesia and mechanical allodynia during the oxaliplatin-induced neuropathy in rats. The treatment with goshajinkigan also exerted significant improvements of oxaliplatin neuropathy in non-resectable or recurrent colorectal cancer patients [129][70]. Moreover, in phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan was shown its benefits towards preventing oxaliplatin-induced neuropathy without affecting the activity of the chemotherapeutic [130][71]. Wei and coworkers[72] [131] monitored the neuroprotective effects of some mixtures of Traditional Chinese Medicine against oxaliplatin in cancer patients. The highest neuroprotective potential had Huangqi Injection which consisted of an extract of Astragalus membranaceus radix and Huangqi Guizhi Wuwu Decoction that contained a mixture of Astragalus membranaceus radix, Cinnamomum cassia, Paeonia lactiflora, Ziziphus jujuba, and Zingiberis recens rhizoma. The common feature for both mixtures was Astragali radix (Huang Qi). The extracts of Astragalus roots, aqueous and two hydroalcoholic extracts, were tested in vitro against oxaliplatin-induced neurotoxicity in the neuronal-derived cell line SH-SY5Y and in primary cultures of rat cortical astrocytes [132][73]. The Astragali radix extracts exhibited strong antioxidant activity, ameliorating the lipid peroxidation, proteins, and DNA oxidation. The 50% hydroalcoholic extract was dominant in the prevention of caspase-3 activation and it stimulates astrocyte viability.
Another immensely important medicinal plant in traditional Chinese medicine is Danshen or Salvia miltiorrhiza, mainly used in the treatment of cardiovascular diseases and neurasthenic insomnia [150][74]. Nevertheless, Danshen and its active constituents tanshinones (tanshinone IIA and cryptotanshinone) exhibited promising activity against oxaliplatin-induced neuropathic pain where they reduced chemotherapy-induced nociceptive hypersensitivity in mice and attenuated glioblastoma cells malignancy. Danshen and tanshinones had the long-lasting pain-relieving effects so they could serve as adjuvant therapy of choice in the oxaliplatin treatment. Another plant tested, because of its significant antioxidant potential, in the model of cisplatin-induced neuropathy in mice, was chamomile (Matricaria chamomilla). The ethanol extract of chamomile flowers showed anti-inflammatory effects and reduction of pain responses in the formalin test in mice with intensive analgesic effect [134][75]. Hypericum perforatum L. (St. John’s wort) is well-known for its antioxidant, anti-inflammatory, analgesic, and neuroprotective effects [151–153][76][77][78]. Its extract showed significant protective activity against oxaliplatin-induced neurotoxicity in vitro and reduced caspase-3 activity in rat astrocytes without the reduction of oxaliplatin cytotoxicity on HT-29 cells [135][79]. Satureja hortensis aerial part extract, applied in rats at three different concentrations (50, 100 and 200 mg/kg) along with cisplatin injection, exhibited strong anxiolytic activity and lowered apoptotic parameters in rat hippocampal tissues [127][68]. Moreover, it was able to significantly reduce the oxidative stress in brain tissues by alleviating CAT and SOD activities and GSH levels and decreasing the levels of lipid peroxidation indicators.
Vitis vinifera L., the common grapevine, is known for many benefits on human health, particularly for the antioxidant potential of its main bioactive compounds proanthocyanidins but also many phenolic acids and flavonoids [106,141]. The
L., the common grapevine, is known for many benefits on human health, particularly for the antioxidant potential of its main bioactive compounds proanthocyanidins but also many phenolic acids and flavonoids[80][50]. The
V. vinifera hydroalcoholic extract in the model of oxaliplatin-induced neurotoxicity showed extensive activity towards the reduction of superoxide anion concentration and lipid peroxidation in rat astrocytes [136]. It also suppressed mechanical and thermal hypersensitivity in rats while a decline of astrocytes activation in the spinal cord was monitored. The grape seed proanthocyanidin extract also showed neuroprotective power against carboplatin in a study reported by Yousef et al. [137]. The main way of acting was through decreasing the oxidative stress in brain tissue and reducing cytokines, p53, neurotransmitters and biochemical parameters. The extract also inhibited brain cell apoptosis and alleviated carboplatin effects on the histological parameters. Green tea (
hydroalcoholic extract in the model of oxaliplatin-induced neurotoxicity showed extensive activity towards the reduction of superoxide anion concentration and lipid peroxidation in rat astrocytes [81]. It also suppressed mechanical and thermal hypersensitivity in rats while a decline of astrocytes activation in the spinal cord was monitored. The grape seed proanthocyanidin extract also showed neuroprotective power against carboplatin in a study reported by Yousef et al. [82]. The main way of acting was through decreasing the oxidative stress in brain tissue and reducing cytokines, p53, neurotransmitters and biochemical parameters. The extract also inhibited brain cell apoptosis and alleviated carboplatin effects on the histological parameters. Green tea (
Camellia sinensis) can attenuate toxicities linked to treatment with platinum-based drugs (cisplatin, oxaliplatin) [154]. Its extract had notable potential on oxaliplatin-induced peripheral neuropathy in rats where caused alleviation of sensory symptoms after oxaliplatin treatment, such as allodynia, but it was inefficient in the prevention of morphometric or electrophysiological alterations [138].
) can attenuate toxicities linked to treatment with platinum-based drugs (cisplatin, oxaliplatin)[83]. Its extract had notable potential on oxaliplatin-induced peripheral neuropathy in rats where caused alleviation of sensory symptoms after oxaliplatin treatment, such as allodynia, but it was inefficient in the prevention of morphometric or electrophysiological alterations[84].
Lithospermum erythrorhizon
is a plant used in traditional Chinese medicine for eczema and other skin diseases as well as for wound healing. However, it was shown that
Lithospermi radix
extract can be an excellent neuroprotective agent against oxaliplatin-induced peripheral neuropathy in both
in vitro and in vivo models [139].
and in vivo models[85].
References
- Galanski, M.; Jakupec, M.; Keppler, B. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094, doi:10.2174/0929867054637626.
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalt. Trans. 2018, 47, 6645–6653, doi:10.1039/c8dt00838h.
- Kosmidis, C.; Sapalidis, K.; Zarogoulidis, P.; Sardeli, C.; Koulouris, C.; Giannakidis, D.; Pavlidis, E.; Katsaounis, A.; Michalopoulos, N.; Mantalobas, S.; et al. Inhaled cisplatin for NSCLC: Facts and results. Int. J. Mol. Sci. 2019, 20, 1–12, doi:10.3390/ijms20082005.
- Bergamini, A.; Pisano, C.; Di Napoli, M.; Arenare, L.; Della Pepa, C.; Tambaro, R.; Facchini, G.; Gargiulo, P.; Rossetti, S.; Mangili, G.; et al. Cisplatin can be safely administered to ovarian cancer patients with hypersensitivity to carboplatin. Gynecol. Oncol. 2017, 144, 72–76, doi:10.1016/j.ygyno.2016.10.023.
- Bucher-Johannessen, C.; Page, C.M.; Haugen, T.B.; Wojewodzic, M.W.; Fosså, S.D.; Grotmol, T.; Haugnes, H.S.; Rounge, T.B. Cisplatin treatment of testicular cancer patients introduces long-term changes in the epigenome. Clin. Epigenetics 2019, 11, 179, doi:10.1186/s13148-019-0764-4.
- Baek, D.W.; Park, J.-Y.; Lee, S.J.; Chae, Y.S. Impressive effect of cisplatin monotherapy on a patient with heavily pretreated triple-negative breast cancer with poor performance. Yeungnam Univ. J. Med. 2020, 37, 230–235, doi:10.12701/yujm.2019.00423.
- Angeli, E.; Nguyen, T.T.; Janin, A.; Bousquet, G. How to make anticancer drugs cross the blood-brain barrier to treat brain metastases. Int. J. Mol. Sci. 2020, 21, doi:10.3390/ijms21010022.
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195, doi:10.1007/s00223-017-0372-2.
- Zhang, L.; Jia, S.; Ma, Y.; Li, L.; Li, X.; Wang, X.; Fu, X.; Ma, W.; Qin, Y.; Li, W.; et al. Efficacy and safety of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) regimen in newly diagnosed, advanced-stage extranodal natural killer/T-cell lymphoma: Interim analysis of a phase 4 study NCT01501149. Oncotarget 2016, 7, 55721–55731, doi:10.18632/oncotarget.10124.
- Nguyen, J.; Solimando, D.A.; Waddell, J.A. Carboplatin and Liposomal Doxorubicin for Ovarian Cancer. Hosp. Pharm. 2016, 51, 442–449, doi:10.1310/hpj5106-442
- Xiang, M.; Colevas, A.D.; Holsinger, F.C.; Le, Q.T.X.; Beadle, B.M. Survival after definitive chemoradiotherapy with concurrent cisplatin or carboplatin for head and neck cancer. JNCCN J. Natl. Compr. Cancer Netw. 2019, 17, 1065–1073, doi:10.6004/jnccn.2019.7297.
- Akamatsu, H.; Ninomiya, K.; Kenmotsu, H.; Morise, M.; Daga, H.; Goto, Y.; Kozuki, T.; Miura, S.; Sasaki, T.; Tamiya, A.; et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV; Springer Singapore, 2019; Vol. 24; ISBN 0123456789.
- Martín-Aragón, T.; Serrano, J.; Benedí, J.; Meiriño, R.M.; García-Alonso, P.; Calvo, F.A. The value of oxaliplatin in the systemic treatment of locally advanced rectal cancer. J. Gastrointest. Oncol. 2018, 9, 631–640, doi:10.21037/jgo.2018.06.02.
- Zhang, F.; Zhang, Y.; Jia, Z.; Wu, H.; Gu, K. Oxaliplatin-Based regimen is superior to cisplatin-Based regimen in tumour remission as first-line chemotherapy for advanced gastric cancer: A meta-analysis. J. Cancer 2019, 10, 1923–1929, doi:10.7150/jca.28896.
- Bogliolo, S.; Cassani, C.; Gardella, B.; Musacchi, V.; Babilonti, L.; Venturini, P.-L.; Ferrero, S.; Spinillo, A. Oxaliplatin for the treatment of ovarian cancer. Expert Opin. Investig. Drugs 2015, 24, 1275–1286, doi:10.1517/13543784.2015.1062874.
- Cao, S.; Wang, C.; Ma, H.; Yin, R.; Zhu, M.; Shen, W.; Dai, J.; Shu, Y.; Xu, L.; Hu, Z.; et al. Genome-wide association study on platinum-induced hepatotoxicity in non-small cell lung cancer patients. Sci. Rep. 2015, 5, 1–8, doi:10.1038/srep11556.
- Ai, D.; Guan, Y.; Liu, X.J.; Zhang, C.F.; Wang, P.; Liang, H.L.; Guo, Q. Sen Clinical comparative investigation of efficacy and toxicity of cisplatin plus gemcitabine or plus abraxane as first-line chemotherapy for stage III/IV non-small-cell lung cancer. Onco. Targets. Ther. 2016, 9, 5693–5698, doi:10.2147/OTT.S109683
- Tixier, F.; Ranchon, F.; Iltis, A.; Vantard, N.; Schwiertz, V.; Bachy, E.; Bouafia-Sauvy, F.; Sarkozy, C.; Tournamille, J.F.; Gyan, E.; et al. Comparative toxicities of 3 platinum-containing chemotherapy regimens in relapsed/refractory lymphoma patients. Hematol. Oncol. 2017, 35, 584–590, doi:10.1002/hon.2328.
- Kart, A.; Cigremis, Y.; Karaman, M.; Ozen, H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 2010, 62, 45–52, doi:10.1016/j.etp.2009.02.066.
- Khan, M. waseem; Zhao, P.; Khan, A.; Raza, F.; Raza, S.M.; Sarfraz, M.; Chen, Y.; Li, M.; Yang, T.; Ma, X.; et al. Synergism of cisplatin-oleanolic acid co-loaded calcium carbonate nanoparticles on hepatocellular carcinoma cells for enhanced apoptosis and reduced hepatotoxicity. Int. J. Nanomedicine 2019, Volume 14, 3753–3771, doi:10.2147/IJN.S196651.
- Eoh, K.J.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, Y.T.; Kim, S.W. Long-term survival analysis of intraperitoneal versus intravenous chemotherapy for primary ovarian cancer and comparison between carboplatin- and cisplatin-based intraperitoneal chemotherapy. J. Korean Med. Sci. 2017, 32, 2021–2028, doi:10.3346/jkms.2017.32.12.2021.
- Grigorian, A.; Brien, C.B.O. Hepatotoxicity Secondary to Chemotherapy. J. Clin. Trans. Hepatol. 2014, 2, 95–102.
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77, doi:10.1016/j.critrevonc.2011.04.012.
- Karavelioglu, E.; Boyaci, M.G.; Simsek, N.; Sonmez, M.A.; Koc, R.; Karademir, M.; Guven, M.; Eser, O. Selenium protects cerebral cells by cisplatin induced neurotoxicity. Acta Cir. Bras. 2015, 30, 394–400, doi:10.1590/S0102-865020150060000004.
- Salman, M.; Naseem, I.; Hassan, I.; Khan, A.A.; Alhazza, I.M. Riboflavin arrests cisplatin-induced neurotoxicity by ameliorating cellular damage in dorsal root ganglion cells. Biomed Res. Int. 2015, 2015, doi:10.1155/2015/603543.
- McDonald, E.S.; Randon, K.R.; Knight, A.; Windebank, A.J. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: A potential mechanism for neurotoxicity. Neurobiol. Dis. 2005, 18, 305–313, doi:10.1016/j.nbd.2004.09.013.
- Podratz, J.L.; Knight, A.M.; Ta, L.E.; Staff, N.P.; Gass, J.M.; Genelin, K.; Schlattau, A.; Lathroum, L.; Windebank, A.J. Cisplatin induced Mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011, 41, 661–668, doi:10.1016/j.nbd.2010.11.017.
- Leo, M.; Schmitt, L.I.; Erkel, M.; Melnikova, M.; Thomale, J.; Hagenacker, T. Cisplatin-induced neuropathic pain is mediated by upregulation of N-type voltage-gated calcium channels in dorsal root ganglion neurons. Exp. Neurol. 2017, 288, 62–74, doi:10.1016/j.expneurol.2016.11.003.
- Zhao, M.; Isami, K.; Nakamura, S.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol. Pain 2012, 8, 1–11, doi:10.1186/1744-8069-8-55.
- Dietrich, J.; Han, R.; Yang, Y.; Mayer-Pröschel, M.; Noble, M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 2006, 5, doi:10.1186/jbiol50.
- Pantic, M.; Minic, M. The Evaluation of the Effects of N-Acetylcysteine on Cisplatin-Induced Alterations in Exploratory Activity in Elevated Plus Maze Test in Rats. Serbian J. Exp. Clin. Res. 2019, 20, 65–72, doi:10.1515/sjecr-2017-0053.
- Abdelkader, N.F.; Saad, M.A.; Abdelsalam, R.M. Neuroprotective effect of nebivolol against cisplatin-associated depressive-like behavior in rats. J. Neurochem. 2017, 141, 449–460, doi:10.1111/jnc.13978.
- Lomeli, N.; Di, K.; Czerniawski, J.; Guzowski, J.F.; Bota, D.A. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic. Biol. Med. 2017, 102, 274–286, doi:10.1016/j.freeradbiomed.2016.11.046.
- Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2003; 149p.
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector. Oncologist 2007, 12, 738–747, doi:10.1634/theoncologist.12-6-738.
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-Induced Neurotoxicity and Preventive Strategies: Past, Present, and Future. Oncologist 2015, 20, 411–432, doi:10.1634/theoncologist.2014-0044.
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up†. Ann. Oncol. 2020, 31. doi:10.1016/j.annonc.2020.07.003.
- Fu, X.; Wu, H.; Li, J.; Wang, C.; Li, M.; Ma, Q.; Yang, W. Efficacy of drug interventions for chemotherapy-induced chronic peripheral neurotoxicity: A network meta-analysis. Front. Neurol. 2017, 8, 4–6, doi:10.3389/fneur.2017.00223.
- Martinez, N.W.; Sánchez, A.; Diaz, P.; Broekhuizen, R.; Godoy, J.; Mondaca, S.; Catenaccio, A.; Macanas, P.; Nervi, B.; Calvo, M.; et al. Metformin protects from oxaliplatin induced peripheral neuropathy in rats. Neurobiol. Pain 2020, 8, 100048, doi:10.1016/j.ynpai.2020.100048.
- Kawashiri, T.; Miyagi, A.; Shimizu, S.; Shigematsu, N.; Kobayashi, D.; Shimazoe, T. Dimethyl fumarate ameliorates chemotherapy agent-induced neurotoxicity in vitro. J. Pharmacol. Sci. 2018, 137, 202–211, doi:10.1016/j.jphs.2018.06.008.
- Areti, A.; Komirishetty, P.; Kumar, A. Carvedilol prevents functional deficits in peripheral nerve mitochondria of rats with oxaliplatin-evoked painful peripheral neuropathy. Toxicol. Appl. Pharmacol. 2017, 322, 97–103. doi:10.1016/j.taap.2017.03.009.
- Akman, T.; Akman, L.; Erbas, O.; Terek, M.C.; Taskiran, D.; Ozsaran, A. The preventive effect of oxytocin to cisplatin-induced neurotoxicity: An experimental rat model. Biomed Res. Int. 2015, 2015, 13–17, doi:10.1155/2015/167235.
- Chiu, G.S.; Maj, M.A.; Rizvi, S.; Dantzer, R.; Vichaya, E.G.; Laumet, G.; Kavelaars, A.; Heijnen, C.J. Pifithrin-m prevents cisplatin-induced chemobrain by preserving neuronal mitochondrial function. Cancer Res. 2017, 77, 742–752, doi:10.1158/0008-5472.CAN-16-1817.
- Chen, X.F.; Wang, R.; Yin, Y.M.; Røe, O.D.; Li, J.; Zhu, L.J.; Guo, R.H.; Wu, T.; Shu, Y.Q. The effect of monosialotetrahexosylganglioside (GM1) in prevention of oxaliplatin induced neurotoxicity: A retrospective study. Biomed. Pharmacother. 2012, 66, 279–284, doi:10.1016/j.biopha.2012.01.002.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803.
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528, doi:10.1039/c8fo01997e.
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.C.; Wong, D.V.T.; Lima-Júnior, R.C.P.; de Albuquerque Ribeiro, R.; Vale, M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 2013, 9, 1–14, doi:10.1186/1744-8069-9-53
- Almutairi, M.M.; Alanazi, W.A.; Alshammari, M.A.; Alotaibi, M.R.; Alhoshani, A.R.; Al-Rejaie, S.S.; Hafez, M.M.; Al-Shabanah, O.A. Neuro-protective effect of rutin against Cisplatin-induced neurotoxic rat model. BMC Complement. Altern. Med. 2017, 17, 1–9, doi:10.1186/s12906-017-1976-9.
- Shahid, M.; Subhan, F.; Ahmad, N.; Sewell, R.D.E. The flavonoid 6-methoxyflavone allays cisplatin-induced neuropathic allodynia and hypoalgesia. Biomed. Pharmacother. 2017, 95, 1725–1733, doi:10.1016/j.biopha.2017.09.108.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892, doi:10.1089/ars.2012.4581.
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513, doi:10.1016/j.foodchem.2014.10.057.
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 1–21, doi:10.3390/nu9050477.
- Domitrović, R.; Potočnjak, I.; Crnčević-Orlić, Ž.; Škoda, M. Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem. Toxicol. 2014, 66, 321–328, doi:10.1016/j.fct.2014.02.002.
- Yüce, A.; Ateşşahin, A.; Ceribaşi, A.O.; Aksakal, M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin. Pharmacol. Toxicol. 2007, 101, 345–349, doi:10.1111/j.1742-7843.2007.00129.x.
- Yamabe, N.; Park, J.Y.; Lee, S.S.; Cho, E.-J.J.; Lee, S.S.; Kang, K.S.; Hwang, G.S.; Kim, S.-N.N.; Kim, H.Y.; Shibamoto, T. Protective effects of protocatechuic acid against cisplatin-induced renal damage in rats. J. Funct. Foods 2015, 19, 20–27, doi:10.1016/j.jff.2015.08.028.
- Chirino, Y.I.; Pedraza-Chaverri, J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009, 61, 223–242, doi:10.1016/j.etp.2008.09.003.
- Li, D.W.; Sun, J.Y.; Wang, K.; Zhang, S.; Hou, Y.J.; Yang, M.F.; Fu, X.Y.; Zhang, Z.Y.; Mao, L.L.; Yuan, H.; et al. Attenuation of Cisplatin-Induced Neurotoxicity by Cyanidin, a Natural Inhibitor of ROS-Mediated Apoptosis in PC12 Cells. Cell. Mol. Neurobiol. 2015, 35, 995–1001, doi:10.1007/s10571-015-0194-6.
- Areti, A.; Komirishetty, P.; Kalvala, A.K.; Nellaiappan, K.; Kumar, A. Rosmarinic Acid Mitigates Mitochondrial Dysfunction and Spinal Glial Activation in Oxaliplatin-induced Peripheral Neuropathy. Mol. Neurobiol. 2018, 55, 7463–7475, doi:10.1007/s12035-018-0920-4.
- Cetin, D.; Hacımuftuoglu, A.; Tatar, A.; Turkez, H.; Togar, B. The in vitro protective effect of salicylic acid against paclitaxel and cisplatin-induced neurotoxicity. Cytotechnology 2016, 68, 1361–1367, doi:10.1007/s10616-015-9896-3.
- Üstün, R.; Oğuz, E.K.; Şeker, A.; Korkaya, H. Thymoquinone prevents cisplatin neurotoxicity in primary DRG neurons. Neurotoxicology 2018, 69, 68–76, doi:10.1016/j.neuro.2018.09.001.
- Kandeil, M.A.; Mahmoud, M.O.; Abdel-Razik, A.R.H.; Gomaa, S.B. Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci. 2019, 228, 145–151, doi:10.1016/j.lfs.2019.04.065.
- Chen, C.; Zhang, H.; Xu, H.; Zheng, Y.; Wu, T.; Lian, Y. Ginsenoside Rb1 ameliorates cisplatin-induced learning and memory impairments. J. Ginseng Res. 2019, 43, 499–507, doi:10.1016/j.jgr.2017.07.009.
- Figueiredo, S.; Filho, S.; Nogueira-Machado, J.; Caligiorne, R. The Anti-Oxidant Properties of Isothiocyanates: A Review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 213–225, doi:10.2174/18722148113079990011.
- Di Cesare Mannelli, L.; Lucarini, E.; Micheli, L.; Mosca, I.; Ambrosino, P.; Soldovieri, M.V.; Martelli, A.; Testai, L.; Taglialatela, M.; Calderone, V.; et al. Effects of natural and synthetic isothiocyanate-based H2S-releasers against chemotherapy-induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology 2017, 121, 49–59, doi:10.1016/j.neuropharm.2017.04.029.
- Lucarini, E.; Micheli, L.; Trallori, E.; Citi, V.; Martelli, A.; Testai, L.; De Nicola, G.R.; Iori, R.; Calderone, V.; Ghelardini, C.; et al. Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2S release in vivo. Phyther. Res. 2018, 32, 2226–2234, doi:10.1002/ptr.6159.
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-induced neuropathy: Oxidative Stress as Pathological Mechanism. Protective Effect of Silibinin. J. Pain 2012, 13, 276–284, doi:10.1016/j.jpain.2011.11.009.
- Lorenzo, J.M.; Putnik, P.; Bursać Kovačević, D.; Petrović, M.; Munekata, P.E.; Gómez, B.; Marszałek, K.; Roohinejad, S.; Barba, F.J. Silymarin compounds: Chemistry, innovative extraction techniques and synthesis. In Studies in Natural Products Chemistry; Elsevier, 2020; pp. 111–130.
- Kumburovic, I.; Selakovic, D.; Juric, T.; Jovicic, N.; Mihailovic, V.; Stankovic, J.K.; Sreckovic, N.; Kumburovic, D.; Jakovljevic, V.; Rosic, G. Antioxidant Effects of Satureja hortensis L. Attenuate the Anxiogenic Effect of Cisplatin in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 8307196, doi:10.1155/2019/8307196.
- Ushio, S.; Egashira, N.; Sada, H.; Kawashiri, T.; Shirahama, M.; Masuguchi, K.; Oishi, R. Goshajinkigan reduces oxaliplatin-induced peripheral neuropathy without affecting anti-tumour efficacy in rodents. Eur. J. Cancer 2012, 48, 1407–1413, doi:10.1016/j.ejca.2011.08.009.
- Nishioka, M.; Shimada, M.; Kurita, N.; Iwata, T.; Morimoto, S.; Yoshikawa, K.; Higashijima, J.; Miyatani, T.; Kono, T. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int. J. Clin. Oncol. 2011, 16, 322–327, doi:10.1007/s10147-010-0183-1.
- Kono, T.; Hata, T.; Morita, S.; Munemoto, Y.; Matsui, T.; Kojima, H.; Takemoto, H.; Fukunaga, M.; Nagata, N.; Shimada, M.; et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin-induced neuropathy. Cancer Chemother. Pharmacol. 2013, 72, 1283–1290, doi:10.1007/s00280-013-2306-7.
- Wei, X.; Zhu, L.; Wang, H.; Wang, C.; Deng, Q.; Li, X. Efficacy of Traditional Chinese Medicines in Preventing Oxaliplatin-induced Peripheral Neurotoxicity in Cancer Patients: A Network Meta-analysis. Chinese Herb. Med. 2017, 9, 161–168, doi:10.1016/s1674-6384(17)60090-x.
- Di Cesare Mannelli, L.; Zanardelli, M.; Bartolucci, G.; Karioti, A.; Bilia, A.R.; Vannacci, A.; Mugelli, A.; Ghelardini, C. In Vitro Evidence for the Use of Astragali Radix Extracts as Adjuvant against Oxaliplatin-Induced Neurotoxicity. Planta Med. 2015, 81, 1045–1055, doi:10.1055/s-0035-1546117.
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30.
- Abad, A.N.A.; Nouri, M.H.K.; Gharjanie, A.; Tavakoli, F. Effect of Matricaria chamomilla Hydroalcoholic Extract on Cisplatin-induced Neuropathy in Mice. Chin. J. Nat. Med. 2011, 9, 126–131, doi:10.3724/SP.J.1009.2011.00126.
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167, doi:10.1016/j.foodchem.2004.03.049.
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, doi:10.3389/fpls.2016.01004.
- Huang, N.; Rizshsky, L.; Hauck, C.; Nikolau, B.J.; Murphy, P.A.; Birt, D.F. Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry 2011, 72, 2015–2023, doi:10.1016/j.phytochem.2011.07.016.
- Cinci, L.; Di Cesare Mannelli, L.; Maidecchi, A.; Mattoli, L.; Ghelardini, C. Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: In vitro evaluations. Zeitschrift fur Naturforsch. C 2017, 72, 219–226, doi:10.1515/znc-2016-0194.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J. Funct. Foods 2015, 18, 820–897, doi:10.1016/j.jff.2015.06.018.
- Micheli, L.; Mattoli, L.; Maidecchi, A.; Pacini, A.; Ghelardini, C.; Di Cesare Mannelli, L. Effect of Vitis vinifera hydroalcoholic extract against oxaliplatin neurotoxicity: in vitro and in vivo evidence. Sci. Rep. 2018, 8, 1–14, doi:10.1038/s41598-018-32691-w.
- Yousef, M.I.; Khalil, D.K.A.M.; Abdou, H.M. Neuro- and nephroprotective effect of grape seed proanthocyanidin extract against carboplatin and thalidomide through modulation of inflammation, tumor suppressor protein p53, neurotransmitters, oxidative stress and histology. Toxicol. Reports 2018, 5, 568–578, doi:10.1016/j.toxrep.2018.04.006.
- Rameshrad, M.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of green tea and its main constituents against natural and chemical toxins: A comprehensive review. Food Chem. Toxicol. 2017, 100, 115–137, doi:10.1016/j.fct.2016.11.035.
- Lee, J.S.; Kim, Y.T.; Jeon, E.K.; Won, H.S.; Cho, Y.S.; Ko, Y.H. Effect of green tea extracts on oxaliplatin-induced peripheral neuropathy in rats. BMC Complement. Altern. Med. 2012, 12, 1, doi:10.1186/1472-6882-12-124.
- Cho, E.S.; Yi, J.M.; Park, J.S.; Lee, Y.J.; Lim, C.J.; Bang, O.S.; Kim, N.S. Aqueous extract of Lithospermi radix attenuates oxaliplatin-induced neurotoxicity in both in vitro and in vivo models. BMC Complement. Altern. Med. 2016, 16, doi:10.1186/s12906-016-1396-2.