Chitin is a nitrogen-enriched polymer abundantly present in the exoskeletons of arthropods, cell walls of fungi, green algae, and microorganisms, radulae and beaks of molluscs and cephalopods, etc. Chitosan is a promising candidate for a wide variety of applications due to its macromolecular structure and its unique biological and physiological properties, including solubility, biocompatibility, biodegradability, and reactivity. Chitosan and its derivatives have been known to be applicable in medicine, pharmaceuticals, food, cosmetics, agriculture, the textile and paper industries, the energy industry, and industrial sustainability. More specifically, their use in drug delivery, dentistry, ophthalmology, wound dressing, cell encapsulation, bioimaging, tissue engineering, food packaging, gelling and coating, food additives and preservatives, active biopolymeric nanofilms, nutraceuticals, skin and hair care, preventing abiotic stress in flora, increasing water availability in plants, controlled release fertilizers, dye-sensitised solar cells, wastewater and sludge treatment, and metal extraction.
- chitosan
- food
- ionic
1. Introduction
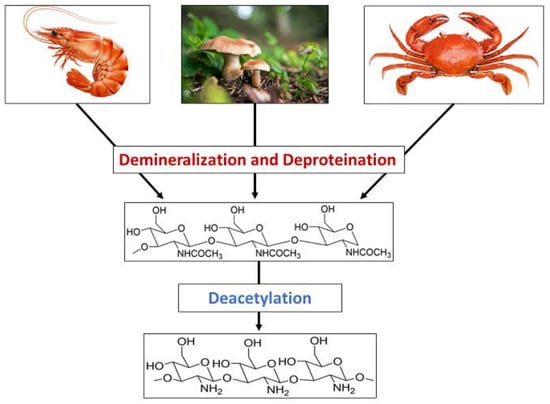
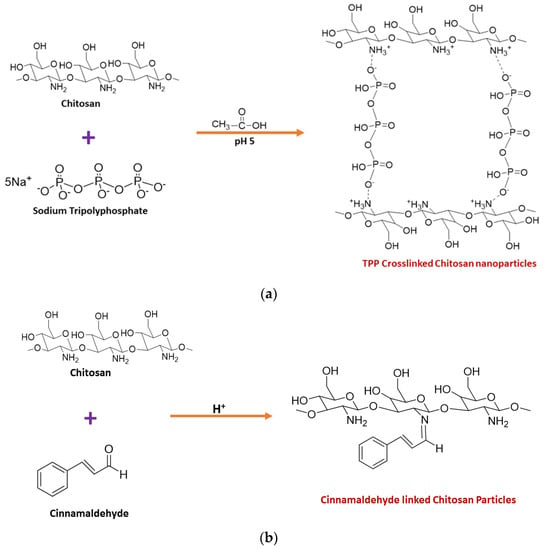
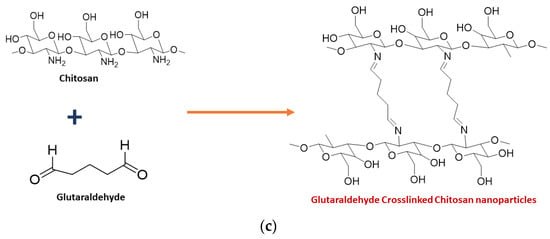
2. Synthesis of Chitosan Nanoparticles
5. Toxicity of Chitosan
Chitosan is a biodegradable polymer whose biodegradability could occur through chemical or enzyme catalysis. The degradability depends on the degree of deacetylation and the availability of amino groups. The toxicity of chitosan depends on the charge density and degree of deacetylation. Kazemi et al. evaluated the cytotoxicity of the thiolated chitosan-lauric acid as a new chitosan derivative by MTT assay. The cytotoxicity of the new polymer and the normal chitosan were not significantly different, as indicated by the cell viability studies performed against normal gingiva human cells (HGF1-PI 1). Chitosan derivative, thiolated chitosan-lauric acid, shows low toxicity due to the intramolecular H bonds, which lead to low flexibility where the rigid structure limits the interactions between the cell membrane and the positive charge of the polymer, rendering it less toxic. On the other hand, lauric acid conjugation to chitosan increases its viability and biocompatibility [330][47]. Chitosan nanogels were found to be free of cytotoxicity when tested against the HEK-293 normal cell line by MTT assay [331][48]. Asghar et al. evaluated the cytotoxicity of green synthesized chitosan coated silver nanoparticles using the HeLa cell line using the MTT assay method and found that 93.2% cell viability was achieved with 100 µg/mL but with higher doses (300 and 400 µg/mL), the viability reduced to 54.5% and 35.2%, respectively. However, the chitosan-coated Ag nanoparticles showed less toxicity compared with Ag nanoparticles, and they exhibit more antibacterial, anticoagulant, antiplatelet, and thrombolytic activities compared with Ag nanoparticles [332][49]. Furthermore, Frigaard et al. reported that among the 25 articles they have referred to except one, all others reported less toxicity of chitosan nanoparticles with >80% cell viability when studied both in vitro and in vivo [333][50], and the only study where low cell viability was reported was the use of dry powder of chitosan nanoparticles (250 and 500 µg/mL) with Calu-6 cells for 24 h [334][51]. Therefore, it is evident that chitosan and its derivatives are less toxic to human cells. As revealed by the examples above throughout the review, it is evident that chitosan is effectively applicable in several fields, and chitosan would be a promising candidate in future applications as well. The discovery and progress of chitosan and its applications are exhibited in Scheme 2.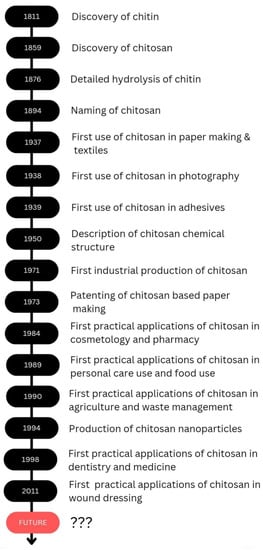
References
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98.
- Seenuvasan, M.; Sarojini, G.; Dineshkumar, M. Recovery of Chitosan from Natural Biotic Waste. Curr. Dev. Biotechnol. Bioeng. Resour. Recover. Wastes 2020, 115–133.
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846.
- Percot, A.; Viton, C.; Domard, A. Characterization of Shrimp Shell Deproteinization. Biomacromolecules 2003, 4, 1380–1385.
- Said Al Hoqani, H.A.; AL-Shaqsi, N.; Hossain, M.A.; Al Sibani, M.A. Isolation and Optimization of the Method for Industrial Production of Chitin and Chitosan from Omani Shrimp Shell. Carbohydr. Res. 2020, 492, 108001.
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.B.; Ahmad, K. Facile Extraction of Chitin and Chitosan from Shrimp Shell. Mater. Today Proc. 2021, 42, 2369–2373.
- Trung, T.S.; Tram, L.H.; Van Tan, N.; Van Hoa, N.; Minh, N.C.; Loc, P.T.; Stevens, W.F. Improved Method for Production of Chitin and Chitosan from Shrimp Shells. Carbohydr. Res. 2020, 489, 107913.
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709.
- Eddya, M.; Tbib, B.; EL-Hami, K. A Comparison of Chitosan Properties after Extraction from Shrimp Shells by Diluted and Concentrated Acids. Heliyon 2020, 6, e03486.
- Li, K.; Guo, Z.; Chen, X.; Wang, J.; El-Araby, A.; El Ghadraoui, L.; Errachidi, F. Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step. Molecules 2022, 27, 8285.
- Pohling, J.; Dave, D.; Liu, Y.; Murphy, W.; Trenholm, S. Two-Step Demineralization of Shrimp (Pandalus borealis) Shells Using Citric Acid: An Environmentally Friendly, Safe and Cost-Effective Alternative to the Traditional Approach. Green Chem. 2022, 24, 1141–1151.
- Younes, I.; Rinaudo, M.; Harding, D.; Sashiwa, H. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Huang, W.C.; Zhao, D.; Xue, C.; Mao, X. An Efficient Method for Chitin Production from Crab Shells by a Natural Deep Eutectic Solvent. Mar. Life Sci. Technol. 2022, 4, 384–388.
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16.
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-Product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130.
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; de Franco, L.O.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290.
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369.
- Mhamdi, S.; Ktari, N.; Hajji, S.; Nasri, M.; Sellami Kamoun, A. Alkaline Proteases from a Newly Isolated Micromonospora Chaiyaphumensis S103: Characterization and Application as a Detergent Additive and for Chitin Extraction from Shrimp Shell Waste. Int. J. Biol. Macromol. 2017, 94, 415–422.
- Hamdi, M.; Hammami, A.; Hajji, S.; Jridi, M.; Nasri, M.; Nasri, R. Chitin Extraction from Blue Crab (Portunus segnis) and Shrimp (Penaeus kerathurus) Shells Using Digestive Alkaline Proteases from P. Segnis Viscera. Int. J. Biol. Macromol. 2017, 101, 455–463.
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leão Gouveia Costa, H.; Martín López, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, Physicochemical Characterization, and Morphological Properties of Chitin and Chitosan from Cuticles of Edible Insects. Food Chem. 2021, 343, 128550.
- Valdez-Peña, A.U.; Espinoza-Perez, J.D.; Sandoval-Fabian, G.C.; Balagurusamy, N.; Hernandez-Rivera, A.; de-la-Garza-Rodriguez, I.M.; Contreras-Esquivel, J.C. Screening of Industrial Enzymes for Deproteinization of Shrimp Head for Chitin Recovery. Food Sci. Biotechnol. 2010, 19, 553–557.
- Lee, D.H.; Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, C.L.; Wang, S.L. Proteases Production and Chitin Preparation from the Liquid Fermentation of Chitinous Fishery By-Products by Paenibacillus Elgii. Mar. Drugs 2021, 19, 477.
- Younes, I.; Ghorbel-Bellaaj, O.; Nasri, R.; Chaabouni, M.; Rinaudo, M.; Nasri, M. Chitin and Chitosan Preparation from Shrimp Shells Using Optimized Enzymatic Deproteinization. Process Biochem. 2012, 47, 2032–2039.
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin Extraction from Shrimp Shell Using Enzymatic Treatment. Antitumor, Antioxidant and Antimicrobial Activities of Chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498.
- Lamarque, G.; Viton, C.; Domard, A. Comparative Study of the First Heterogeneous Deacetylation of α- and β-Chitins in a Multistep Process. Biomacromolecules 2004, 5, 992–1001.
- Harmsen, R.A.G.; Tuveng, T.R.; Antonsen, S.G.; Eijsink, V.G.H.; Sørlie, M. Can We Make Chitosan by Enzymatic Deacetylation of Chitin? Molecules 2019, 24, 3862.
- Yang, G.; Hou, X.; Lu, J.; Wang, M.; Wang, Y.; Huang, Y.; Liu, Q.; Liu, S.; Fang, Y. Enzymatic Modification of Native Chitin and Chitin Oligosaccharides by an Alkaline Chitin Deacetylase from Microbacterium Esteraromaticum MCDA02. Int. J. Biol. Macromol. 2022, 203, 671–678.
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243.
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189.
- Sullivan, D.J.; Cruz-Romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Synthesis of Monodisperse Chitosan Nanoparticles. Food Hydrocoll. 2018, 83, 355–364.
- Othman, N.; Masarudin, M.J.; Kuen, C.Y.; Dasuan, N.A.; Abdullah, L.C.; Jamil, S.N.A.M. Synthesis and Optimization of Chitosan Nanoparticles Loaded with L-Ascorbic Acid and Thymoquinone. Nanomaterials 2018, 8, 920.
- Ghadi, A.; Mahjoub, S.; Tabandeh, F.; Talebnia, F. Synthesis and Optimization of Chitosan Nanoparticles: Potential Applications in Nanomedicine and Biomedical Engineering. Casp. J. Intern. Med. 2014, 5, 156.
- Liu, G.; Shao, L.; Ge, F.; Chen, J. Preparation of Ultrafine Chitosan Particles by Reverse Microemulsion. China Particuology 2007, 5, 384–390.
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as Nanoreactors for Synthesis of Biopolymer Nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130.
- Wu, Y.; Wang, Y.; Luo, G.; Dai, Y. Effect of Solvents and Precipitant on the Properties of Chitosan Nanoparticles in a Water-in-Oil Microemulsion and Its Lipase Immobilization Performance. Bioresour. Technol. 2010, 101, 841–844.
- Pandey, P.; Dua, K.; Dureja, H. Erlotinib Loaded Chitosan Nanoparticles: Formulation, Physicochemical Characterization and Cytotoxic Potential. Int. J. Biol. Macromol. 2019, 139, 1304–1316.
- Yurtdaş Kirimlioğlu, G.; Öztürk, A.A. Levocetirizine Dihydrochloride-Loaded Chitosan Nanoparticles: Formulation and In Vitro Evaluation. TURKISH J. Pharm. Sci. 2020, 17, 27–35.
- Gover Antoniraj, M.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Cross-Linked Chitosan Microparticles Preparation by Modified Three Fluid Nozzle Spray Drying Approach. Int. J. Biol. Macromol. 2020, 147, 1268–1277.
- Orellano, M.S.; Longo, G.S.; Porporatto, C.; Correa, N.M.; Falcone, R.D. Role of Micellar Interface in the Synthesis of Chitosan Nanoparticles Formulated by Reverse Micellar Method. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 599, 124876.
- Orellano, M.S.; Isaac, P.; Breser, M.L.; Bohl, L.P.; Conesa, A.; Falcone, R.D.; Porporatto, C. Chitosan Nanoparticles Enhance the Antibacterial Activity of the Native Polymer against Bovine Mastitis Pathogens. Carbohydr. Polym. 2019, 213, 1–9.
- El-Naggar, N.E.A.; Saber, W.E.I.A.; Zweil, A.M.; Bashir, S.I. An Innovative Green Synthesis Approach of Chitosan Nanoparticles and Their Inhibitory Activity against Phytopathogenic Botrytis Cinerea on Strawberry Leaves. Sci. Rep. 2022, 12, 1–20.
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green Synthesis of Chitosan-Cinnamaldehyde Cross-Linked Nanoparticles: Characterization and Antibacterial Activity. Carbohydr. Polym. 2019, 226, 115298.
- Galan, J.; Trilleras, J.; Zapata, P.A.; Arana, V.A.; Grande-Tovar, C.D. Optimization of Chitosan Glutaraldehyde-Crosslinked Beads for Reactive Blue 4 Anionic Dye Removal Using a Surface Response Methodology. Life 2021, 11, 85.
- Duraisamy, N.; Dhayalan, S.; Shaik, M.R.; Shaik, A.H.; Shaik, J.P.; Shaik, B. Green Synthesis of Chitosan Nanoparticles Using of Martynia annua L. Ethanol Leaf Extract Their Antibacterial Activity. Crystals 2022, 12, 1550.
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Jeevithan, E.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Fungal Enzyme-Mediated Synthesis of Chitosan Nanoparticles and Its Biocompatibility, Antioxidant and Bactericidal Properties. Int. J. Biol. Macromol. 2018, 118, 1542–1549.
- Tuorkey, M.; Khedr, Y.; Aborhyem, S.; Xue, X. Green Synthesis of Chicory (Cichorium intybus L.) Chitosan Nanoparticles and Evaluation of Their Anti-Fungal, Anti-Hemolytic, and Anti-Cancer Activities. J. Bioact. Compat. Polym. 2022, 37, 421–436.
- Kazemi, M.S.; Mohammadi, Z.; Amini, M.; Yousefi, M.; Tarighi, P.; Eftekhari, S.; Rafiee Tehrani, M. Thiolated Chitosan-Lauric Acid as a New Chitosan Derivative: Synthesis, Characterization and Cytotoxicity. Int. J. Biol. Macromol. 2019, 136, 823–830.
- Piri-Gharaghie, T.; Beiranvand, S.; Riahi, A.; Shirin, N.J.; Badmasti, F.; Mirzaie, A.; Elahianfar, Y.; Ghahari, S.; Ghahari, S.; Pasban, K.; et al. Fabrication and Characterization of Thymol-Loaded Chitosan Nanogels: Improved Antibacterial and Anti-Biofilm Activities with Negligible Cytotoxicity. Chem. Biodivers. 2022, 19, e202100426.
- Asghar, M.A.; Yousuf, R.I.; Shoaib, M.H.; Asghar, M.A. Antibacterial, Anticoagulant and Cytotoxic Evaluation of Biocompatible Nanocomposite of Chitosan Loaded Green Synthesized Bioinspired Silver Nanoparticles. Int. J. Biol. Macromol. 2020, 160, 934–943.
- Frigaard, J.; Jensen, J.L.; Galtung, H.K.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An Overview of the Cytotoxicity of Chitosan Based Nanoparticles. Front. Pharmacol. 2022, 13, 1492.
- Dehghan, S.; Kheiri, M.T.; Tabatabaiean, M.; Darzi, S.; Tafaghodi, M. Dry-Powder Form of Chitosan Nanospheres Containing Influenza Virus and Adjuvants for Nasal Immunization. Arch. Pharm. Res. 2013, 36, 981–992.
