Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Fouad Khalil.
Left ventricular assist devices (LVADs) have been increasingly used in patients with advanced heart failure, either as a destination therapy or as a bridge to heart transplant. Continuous flow (CF) LVADs have revolutionized advanced heart failure treatment. However, gastrointestinal bleeding (GIB) remain a major concern in this patient population. This entry provides an overview of the pathophysiology, clinical implications and management of GIB in patients with LVAD.
- continuous flow left ventricular assist device
- gastrointestinal bleeding
- stroke
1. Introduction
Left ventricular assist device (LVAD) support has become a valuable therapeutic option to improve survival and quality of life in patients with advanced heart failure [1]. Despite the advancements in LVAD design, long-term exposure to continuous-flow (CF) LVADs has been linked to vascular dysfunction and major vascular sequelae, including bleeding and thrombosis. The endothelium plays a major role in vascular dysfunction exhibited by CF-LVAD recipients. The reduced pulsatility in CF-LVADs is thought to be a major factor for endothelial dysfunction. Moreover, supraphysiological shear stress in CF-LVADs results in hemolysis, von Willebrand factor (VWF) degradation and other changes that eventually contribute to the development of vascular pathology [2,3,4,5][2][3][4][5]. The non-physiological flow pattern in CF-LVADs worsens the endothelial dysfunction and results in elevated reactive oxygen species, generation of proinflammatory factors, platelet activation, vascular wall permeability, dysregulated vascular tone, and nitric oxide (NO) deficiency [6]. These changes in the vascular bed lead to several vascular complications that can impact the quality of life, heart transplant (HT) probability, and survival in CF-LVAD patients.
2. Impact of CF-LVAD on Gastrointestinal Vasculature and GIB
VWF plays a vital role in pathophysiology of GIB (Figure 21). The attenuated pulsatility in CF-LVAD seems to result in direct inhibition of VWF secretion by endothelial cells and indirect inhibition by reducing endothelial NO secretion that leads to negative-feedback inhibition of VWF secretion [30][7].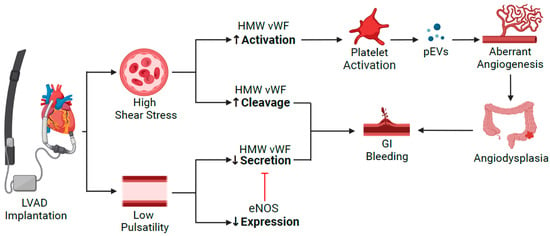
Figure 21. Pathophysiology of GIB in patients supported with CF-LVAD. VWF deficiency is the common pathway for development of angiodysplasia due to the high shear stress and continuous flow. eNOS: endothelial nitric oxide synthase; GI: gastrointestinal; HMW VWF: high molecular weight von Willebrand Factor; pEVs: platelet-derived extracellular vesicles.
2.1. Clinical Perspective
Despite the beneficial role of LVADs in patients with advanced heart failure, GIB is one of the major complications in this patient population [40,41][17][18]. The reported incidence of GIB post-CF-LVAD implantation ranges from 21% to 31% [42,43][19][20]. Multiple studies of GIB in LVADs reported that the majority of cases had therapeutic or subtherapeutic INR levels at the time of bleeding [43][20]. Predictors of GIB include older age, redo sternotomy, preoperative inotrope use, elevated preoperative creatinine, RV failure, and concomitant antiplatelet and anticoagulant use [42][19]. Upper GIB seems to be the main location of bleeding in LVAD patients with AVM, with angiodysplasia being the most common culprit [43,44][20][21]. In a pooled analysis of 1087 patients, the mean duration from CF-LVAD implantation to first bleeding event was 54 days, and anemia was the most common presentation, followed by melena [45][22]. The occurrence of GIB is associated with increased morbidity and mortality. Moreover, the need for repeated blood transfusions increases alloimmunization risk, which may limit HT offers [46][23]. This prompts the need to develop treatment strategies to prevent GIB.2.2. Interventions and Medications to Reduce GIB in CF-LVAD-Implanted Patients
2.2.1. Pump Design
The HM 3 device is recognized as a fully magnetically levitated device that has the potential to reduce shear stress, and it provides artificial pulsatility. Notwithstanding, these design changes did not lead to lower GIB when compared to HM 2 in the momentum trial [47][24]. Netuka et al. documented an 18% decrease in VWF with the HM 3 device, compared with a 46% to 73% reduction with the HM II device, after 45 days of support, with no measurable differences in ADAMTS-13 activity levels [48][25]. This suggests that the HM 3 device may still induce mechanical shear stress adequate to disturb VWF homeostasis. Furthermore, it seems that the HM 3 device likely provides an arterial pulsatility below the physiologic minimum level needed to reduce bleeding events. The markedly lower pump thrombosis rates with newer CF-LVADs, particularly the HM 3 LVAD, has prompted discussion regarding the potential for avoidance of antiplatelet therapy to reduce bleeding risk based on observation data showing lower risk of re-bleeding without increase in thrombotic complications after discontinuation of aspirin in HM2 and HM3 LVAD patients [49,50,51,52][26][27][28][29]. However, the feasibility of such a strategy may be device-specific, as an Aspirin dose of 81 mg daily instead of 325 mg daily was associated with increased risk of thrombosis for the HeartWare HVAD pump, which was not the case for the HM3 and HM 2 devices [53,54,55,56][30][31][32][33].2.2.2. Medical Management
There is limited data regarding the potential benefit of pharmacological interventions, most of which comes from observational studies in patients with recurrent or refractory LVAD-related GIB. It has been hypothesized that angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs) may reduce angiogenesis by inhibiting angiotensin II-related activation of the transforming growth factor beta (TGF-β) and VEGF pathways. A recent systematic review and meta-analysis by Kittipibul et al. found that retrospective data from 3 studies [57,58,59][34][35][36] with a total of 619 CF-LVAD patients showed ACEi/ARB use was associated with a decreased incidence of overall GIB [60][37]. Interestingly, while there was a trend towards reduced odds of AVM-related GIB with ACEI/ARB use, it was not statistically significant as the largest study by Shultz et al. with 377 patients found no significant difference in AVM-related GIB rates by ACEi/ARB usage [58][35]. The protective effect seems to be seen with a dose threshold of >5 mg daily lisinopril equivalence rather than being dose-dependent [59][36] and seems independent of BP effect [57][34]. Unfortunately, the definitions of ACEI/ARB usage and GIB events vary amongst these studies. Furthermore, there is conflicting data showing no significant association between GIB risk and ACEi/ARB use in a retrospective analysis using data from 13,732 patients in the INTERMACS registry, about 52% of whom were on ACEi/ARB [61][38]. Interestingly, this analysis showed a lower risk of GIB in patients on beta blockers [61][38], which was not the case in the smaller study by Houston et al. [57][34]. The anti-VEGF monoclonal antibody, bevacizumab, was well tolerated and markedly reduced the need for transfusions, endoscopies, and GIB-related hospitalizations in a small pilot study involving five HM II LVAD patients with refractory angiodysplasia-related GIB over a median follow-up period of 22 months [62][39]. The somatostatin analog, octreotide, lowers portal pressures by splanchnic vasoconstriction and downregulates VEGF and basic fibroblast growth factor to inhibit angiogenesis, and it has been used in variceal bleeding as well as non-variceal angiodysplasia-related GIB [63][40]. It is well tolerated, with most of the available data from small observational studies showing some potential benefit in reducing GIB recurrence in CF-LVAD patients [63,64,65,66,67,68,69,70][40][41][42][43][44][45][46][47]. The data regarding digoxin’s potential role in LVAD-related GIB management is inconclusive. There are a few retrospective studies linking digoxin use to significant reduction in all-cause GIB, particularly in angiodysplasia-related GIB in CF-LVAD patients [71,72,73][48][49][50]. The proposed mechanism is suppression of hypoxia-inducible factor-1 α (HIF-1α), a mediator of angiopoietin-2-induced angiodysplasia [71][48]. However, a large retrospective analysis using the INTERMACS database by Jennings et al. in 2020, with over 2000 CF-LVAD patients on digoxin, found no association between LVAD-related GIB rates and digoxin use [61][38]. There is very limited data regarding the use of hormone therapy in CF-LVAD-related GIB. A small single-center proof-of-concept retrospective observational study has suggested significant reduction in GIB-related transfusions and hospitalizations with danazol use [74][51]. There is conflicting data regarding estrogen-based hormone therapy for AVM-related GIB prevention, and the potential for increased thromboembolic risk poses reservations. Thalidomide is thought to downregulate HIF-1α expression and inhibit VEGF and basic fibroblast factor [75,76,77,78][52][53][54][55]. Its antiangiogenic properties have shown some promise in refractory angiodysplasia-related GIB, including in LVAD patients [78,79,80,81,82][55][56][57][58][59]. The largest retrospective study to date showed that thalidomide use in 17 CF-LVAD patients with angiodysplasia-related GIB was found to significantly reduce the risk of rebleed, median number of GIBs per year, and transfusion requirements per year while on thalidomide versus while off thalidomide (before initiation) [78][55]. Adverse event rate was 59%, albeit with dose reduction resolving symptoms in most patients without increased GIB [78][55]. Barriers to its use include high incidence of adverse effects which seem to be dose related, with unclear minimal effective dose, and the need for provider and pharmacist enrollment in the THALOMID Risk Evaluation and Mitigation Strategy (REMS) program to prescribe thalidomide due to teratogenicity. Desmopressin is a vasopressin analog currently used to treat Hemophilia A and von Willibrand disease. It shortens bleeding time and improves hemostasis by increasing VWF and factor VIII levels, making it an attractive potential therapy for the acquired VWF deficiency implicated in LVAD-related GIB. In one case report, desmopressin prevented rebleeding for 6 months in one HM II LVAD patient with refractory GIB, despite holding antithrombotic therapies and starting octreotide [83][60]. The data is inadequate to provide a recommendation, as further studies are needed to determine efficacy and safety given potential for hyponatremia, fluid retention, and thrombosis.References
- McNamara, N.; Narroway, H.; Williams, M.; Brookes, J.; Farag, J.; Cistulli, D.; Bannon, P.; Marasco, S.; Potapov, E.; Loforte, A. Contemporary outcomes of continuous-flow left ventricular assist devices—A systematic review. Ann. Cardiothorac. Surg. 2021, 10, 186–208.
- De Waal, E.E.; Van Zaane, B.; Van Der Schoot, M.M.; Huisman, A.; Ramjankhan, F.; Van Klei, W.A.; Marczin, N. Vasoplegia after implantation of a continuous flow left ventricular assist device: Incidence, outcomes and predictors. BMC Anesthesiol. 2018, 18, 1–12.
- Ambardekar, A.v.; Hunter, K.S.; Babu, A.N.; Tuder, R.M.; Dodson, R.B.; Lindenfeld, J. Changes in Aortic Wall Structure, Composition, and Stiffness With Continuous-Flow Left Ventricular Assist Devices: A Pilot Study. Circ. Heart Fail. 2015, 8, 944–952.
- Bartoli, C.R.; Restle, D.J.; Zhang, D.M.; Acker, M.A.; Atluri, P. Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: Mechanical demolition and enzymatic cleavage. J. Thorac. Cardiovasc. Surg. 2015, 149, 281–289.
- Amir, O.; Radovancevic, B.; Delgado, R.M.; Kar, B.; Radovancevic, R.; Henderson, M.; Cohn, W.E.; Smart, F.W. Peripheral Vascular Reactivity in Patients With Pulsatile vs. Axial Flow Left Ventricular Assist Device Support. J. Heart Lung Transplant. 2006, 25, 391–394.
- Poredos, P.; Jezovnik, M.K.; Radovancevic, R.; Gregoric, I.D. Endothelial Function in Patients With Continuous-Flow Left Ventricular Assist Devices. Angiology 2020, 72, 9–15.
- Hydren, J.R.; Richardson, R.S.; Wever-Pinzon, O.; Drakos, S.G. The “double whammy” of a continuous-flow left ventricular assist device on von Willebrand factor. J. Thorac. Cardiovasc. Surg. 2020, 159, 910–915.
- Wang, Y.; Nguyen, K.T.; Ismail, E.; Donoghue, L.; Giridharan, G.A.; Sethu, P.; Cheng, X. Effect of pulsatility on shear-induced extensional behavior of Von Willebrand factor. Artif. Organs 2021, 46, 887–898.
- Malehsa, D.; Meyer, A.L.; Bara, C.; Strüber, M. Acquired von Willebrand syndrome after exchange of the HeartMate XVE to the HeartMate II ventricular assist device. Eur. J. Cardio-Thoracic Surg. 2009, 35, 1091–1093.
- Michiels, J.J.; Berneman, Z.; Gadisseur, A.; van der Planken, M.; Schroyens, W.; Van de Velde, A.; van Vliet, H. Classification and Characteri-zation of Hereditary Types 2A, 2B, 2C, 2D, 2E, 2M, 2N, and 2U (Unclassifiable) von Willebrand Disease. Clin. Appl. Thromb. Hemost. 2016, 12, 397–420.
- Uriel, N.; Pak, S.W.; Jorde, U.P.; Jude, B.; Susen, S.; Vincentelli, A.; Ennezat, P.V.; Cappleman, S.; Naka, Y.; Mancini, D. Acquired von Willebrand Syndrome After Continuous-Flow Mechanical Device Support Contributes to a High Prevalence of Bleeding During Long-Term Support and at the Time of Trans-plantation. J. Am. Coll. Cardiol. 2010, 56, 1207–1213.
- Tabit, C.E.; Chen, P.; Kim, G.H.; Fedson, S.E.; Sayer, G.; Coplan, M.J.; Jeevanandam, V.; Uriel, N.; Liao, J.K. Elevated Angiopoietin-2 Level in Patients with Continu-ous-Flow Left Ventricular Assist Devices Leads to Altered Angiogenesis and Is Associated with Higher Nonsurgical Bleeding. Circulation 2016, 134, 141–152.
- Patel, S.R.; Madan, S.; Saeed, O.; Algodi, M.; Luke, A.; Gibber, M.; Goldstein, D.J.; Jorde, U.P. Association of Nasal Mucosal Vascular Alterations, Gastroin-testinal Arteriovenous Malformations, and Bleeding in Patients With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail. 2016, 4, 962–970.
- Starke, R.D.; Ferraro, F.; Paschalaki, K.; Dryden, N.H.; McKinnon, T.A.J.; Sutton, R.E.; Payne, E.M.; Haskard, D.O.; Hughes, A.; Cutler, D.; et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011, 117, 1071–1080.
- Selvam, S.; James, P. Angiodysplasia in von Willebrand Disease: Understanding the Clinical and Basic Science. Semin. Thromb. Hemost. 2017, 43, 572–580.
- Yang, M.; Houck, K.L.; Dong, X.; Hernandez, M.; Wang, Y.; Nathan, S.S.; Wu, X.; Afshar-Kharghan, V.; Fu, X.; Cruz, M.A.; et al. Hyperadhesive von Willebrand Factor Promotes Ex-tracellular Vesicle-Induced Angiogenesis: Implication for LVAD-Induced Bleeding. Basic Transl. Sci. 2022, 7, 247–261.
- Crow, S.; John, R.; Boyle, A.; Shumway, S.; Liao, K.; Colvin-Adams, M.; Toninato, C.; Missov, E.; Pritzker, M.; Martin, C.; et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2009, 137, 208–215.
- Genovese, E.A.; Dew, M.A.; Teuteberg, J.J.; Simon, M.A.; Kay, J.; Siegenthaler, M.P.; Bhama, J.K.; Bermudez, C.A.; Lockard, K.L.; Winowich, S.; et al. Incidence and Patterns of Adverse Event Onset During the First 60 Days After Ventricular Assist Device Implantation. Ann. Thorac. Surg. 2009, 88, 1162–1170.
- Stulak, J.M.; Davis, M.E.; Haglund, N.; Dunlay, S.; Cowger, J.; Shah, P.; Pagani, F.D.; Aaronson, K.D.; Maltais, S. Adverse events in contemporary continuous-flow left ventricular assist devices: A multi-institutional comparison shows significant differences. J. Thorac. Cardiovasc. Surg. 2015, 151, 177–189.
- Draper, K.V.; Huang, R.J.; Gerson, L.B. GI bleeding in patients with continuous-flow left ventricular assist devices: A systematic review and meta-analysis. Gastroint. Endosc. 2014, 80, 435–446.e1.
- Kang, J.; Hennessy-Strahs, S.; Kwiatkowski, P.; Bermudez, C.A.; Acker, M.A.; Atluri, P.; McConnell, P.I.; Bartoli, C.R. Continuous-Flow LVAD Support Causes a Distinct Form of Intestinal Angiodysplasia. Circ. Res. 2017, 121, 963–969.
- Carlson, L.A.; Maynes, E.J.; Choi, J.H.; Hallett, A.M.; Horan, D.P.; Weber, M.P.; Deb, A.K.; Patel, S.; Samuels, L.E.; Morris, R.J.; et al. Characteristics and outcomes of gastrointestinal bleeding in patients with continuous-flow left ventricular assist devices: A systematic review. Artif. Organs 2020, 44, 1150–1161.
- Holley, C.T.; Harvey, L.; Roy, S.S.; Cogswell, R.; Eckman, P.; Liao, K.; John, R. Gastrointestinal bleeding during continuous-flow left ven-tricular assist device support is associated with lower rates of cardiac transplantation. ASAIO J. 2015, 61, 635–639.
- Mehra, M.R.; Cleveland, J.C.; Uriel, N.; Cowger, J.A.; Hall, S.; Horstmanshof, D.; Naka, Y.; Salerno, C.T.; Chuang, J.; Williams, C.; et al. Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: A study of 2200 HeartMate 3 left ventricular assist device implants. Eur. J. Heart Fail. 2021, 23, 1392–1400.
- Netuka, I.; Kvasnička, T.; Kvasnička, J.; Hrachovinová, I.; Ivák, P.; Mareček, F.; Bílková, J.; Malíková, I.; Jančová, M.; Malý, J.; et al. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J. Heart Lung Transplant. 2016, 35, 860–867.
- Katz, J.N.; Adamson, R.M.; John, R.; Tatooles, A.; Sundareswaran, K.; Kallel, F.; Farrar, D.J.; Jorde, U.P. Safety of reduced anti-thrombotic strategies in HeartMate II patients: A one-year analysis of the US-TRACE Study. J. Heart Lung Transplant. 2015, 34, 1542–1548.
- Netuka, I.; Litzler, P.-Y.; Berchtold-Herz, M.; Flecher, E.; Zimpfer, D.; Damme, L.; Sundareswaran, K.S.; Farrar, D.J.; Schmitto, J.D. Outcomes in HeartMate II Patients With No Antiplatelet Therapy: 2-Year Results From the European TRACE Study. Ann. Thorac. Surg. 2016, 103, 1262–1268.
- Lim, H.S.; Ranasinghe, A.; Chue, C.; Mascaro, J. Two-year outcome of warfarin monotherapy in HeartMate 3 left ventricular assist device: A single-center experience. J. Heart Lung Transplant. 2020, 39, 1149–1151.
- Consolo, F.; Raimondi Lucchetti, M.; Tramontin, C.; Lapenna, E.; Pappalardo, F. Do we need aspirin in HeartMate 3 patients? Eur. J. Heart Fail. 2019, 21, 815–817.
- Mehra, M.R.; Crandall, D.L.; Gustafsson, F.; Jorde, U.P.; Katz, J.N.; Netuka, I.; Uriel, N.; Connors, J.M.; Sood, P.; Heatley, G.; et al. Aspirin and left ventricular assist devices: Rationale and design for the international randomized, placebo-controlled, non-inferiority ARIES HM3 trial. Eur. J. Heart Fail. 2021, 23, 1226–1237.
- Najjar, S.S.; Slaughter, M.S.; Pagani, F.D.; Starling, R.C.; McGee, E.C.; Eckman, P.; Tatooles, A.J.; Moazami, N.; Kormos, R.L.; Hathaway, D.R.; et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J. Heart Lung Transplant. 2013, 33, 23–34.
- Slaughter, M.S.; Pagani, F.D.; McGee, E.C.; Birks, E.J.; Cotts, W.G.; Gregoric, I.; Frazier, O.H.; Icenogle, T.; Najjar, S.S.; Boyce, S.W.; et al. HeartWare ventricular assist system for bridge to transplant: Combined results of the bridge to transplant and continued access protocol trial. J. Heart Lung Transplant. 2013, 32, 675–683.
- Saeed, O.; Colombo, P.C.; Mehra, M.R.; Uriel, N.; Goldstein, D.J.; Cleveland, J.; Connors, J.M.; Najjar, S.S.; Mokadam, N.A.; Bansal, A.; et al. Effect of aspirin dose on hemocompatibility-related outcomes with a magnetically levitated left ventricular assist device: An analysis from the MOMENTUM 3 study. J. Heart Lung Transplant. 2020, 39, 518–525.
- Houston, B.A.; Schneider, A.L.C.; Vaishnav, J.; Cromwell, D.M.; Miller, P.E.; Faridi, K.F.; Shah, A.; Sciortino, C.; Whitman, G.; Tedford, R.J.; et al. Angiotensin II antagonism is associated with reduced risk for gastrointestinal bleeding caused by arteriovenous malformations in patients with left ventricular assist devices. J. Heart Lung Transplant. 2016, 36, 380–385.
- Schultz, J.; John, R.; Alexy, T.; Thenappan, T.; Cogswell, R. Association between angiotensin II antagonism and gastrointestinal bleeding on left ventricular assist device support. J. Heart Lung Transplant. 2018, 38, 469–471.
- Converse, M.P.; Sobhanian, M.; Taber, D.J.; Houston, B.A.; Meadows, H.B.; Uber, W.E. Effect of Angiotensin II Inhibitors on Gastroin-testinal Bleeding in Patients With Left Ventricular Assist Devices. J. Am. Coll Cardiol. 2019, 73, 1769–1778.
- Kittipibul, V.; Vutthikraivit, W.; Kewcharoen, J.; Rattanawong, P.; Tantrachoti, P.; Putthapiban, P.; Nair, N. Angiotensin II antagonists and gastrointestinal bleeding in left ventricular assist devices: A systematic review and meta-analysis. Int. J. Artif. Organs 2020, 44, 215–220.
- Jennings, D.L.; Truby, L.K.; Littlefield, A.J.; Ciolek, A.M.; Marshall, D.; Jain, R.; Topkara, V.K. Impact of heart failure drug therapy on rates of gastrointestinal bleeding in LVAD recipients: An INTERMACS analysis. Int. J. Artif. Organs 2021, 44, 965–971.
- Asleh, R.; Albitar, H.A.H.; Schettle, S.D.; Kushwaha, S.S.; Pereira, N.L.; Behfar, A.; Stulak, J.M.; Rodeheffer, R.J.; Iyer, V.N. Intravenous bevacizumab as a novel treatment for refractory left ventricular assist device-related gastrointestinal bleeding. J. Heart Lung Transplant. 2020, 39, 492–495.
- Del Rio-Pertuz, G.; Nair, N. Gastrointestinal bleeding in patients with continuous-flow left ventricular assist devices: A comprehensive review. Artif. Organs 2022, 47, 12–23.
- Littlefield, A.J.; Jones, G.; Ciolek, A.M.; Yuzefpolskaya, M.; Jennings, D.L. A reappraisal of the pharmacologic management of gastro-intestinal bleeding in patients with continuous flow left ventricular assist devices. Heart Fail Rev. 2021, 26, 277–288.
- Aggarwal, A.; Pant, R.; Kumar, S.; Sharma, P.; Gallagher, C.; Tatooles, A.J.; Pappas, P.S.; Bhat, G. Incidence and Management of Gastrointestinal Bleeding With Continuous Flow Assist Devices. Ann. Thorac. Surg. 2012, 93, 1534–1540.
- Shah, K.B.; Gunda, S.; Emani, S.; Kanwar, M.K.; Uriel, N.; Colombo, P.C.; Uber, P.A.; Sears, M.L.; Chuang, J.; Farrar, D.J.; et al. Multicenter Evaluation of Octreotide as Secondary Prophylaxis in Patients With Left Ventricular Assist Devices and Gastrointestinal Bleeding. Circ. Heart Fail. 2017, 10, e004500.
- Wilson, T.J.; Baran, D.A.; Herre, J.M.; Cameron, C.M.; Yehya, A.; Ingemi, A.I. Gastrointestinal Bleeding Rates in Left Ventricular Assist Device Population Reduced with Octreotide Utilization. ASAIO J. 2020.
- Juricek, C.; Imamura, T.; Nguyen, A.; Chung, B.; Rodgers, D.; Sarswat, N.; Kim, G.; Raikhelkar, J.; Ota, T.; Song, T.; et al. Long-Acting Octreotide Reduces the Recurrence of Gastrointestinal Bleeding in Patients With a Continuous-Flow Left Ventricular Assist Device. J. Card. Fail. 2018, 24, 249–254.
- Malhotra, R.; Shah, K.B.; Chawla, R.; Pedram, S.; Smallfield, M.C.; Priday, A.G.; DeWilde, C.T.; Brophy, D.F. Tolerability and Biological Effects of Long-Acting Octreotide in Patients With Continuous Flow Left Ventricular Assist Devices. ASAIO J. 2017, 63, 367–370.
- Smallfield, G.; Gunda, S.; Emani, S.; Kanwar, M.; Uriel, N.; Colombo, P.; Uber, P.; Sears, M.; Shah, K. A Multicenter Evaluation of Octreotide for Ventricular Assist Device Related Gastrointestinal Bleeding. J. Heart Lung Transplant. 2016, 35, S245.
- Vukelic, S.; Vlismas, P.P.; Patel, S.R.; Xue, X.; Shitole, S.G.; Saeed, O.; Sims, D.B.; Chinnadurai, T.; Shin, J.J.; Forest, S.J.; et al. Digoxin Is Associated With a Decreased Incidence of An-giodysplasia-Related Gastrointestinal Bleeding in Patients With Continuous-Flow Left Ventricular Assist Devices. Circ. Heart Fail. 2018, 11, e004899.
- Abbasi, M.A.; A Stoller, D.; Lyden, E.; Lowes, B.D.; Zolty, R.; Lundgren, S.W. Impact of digoxin utilization on clinical outcomes following left ventricular assist device implantation. Int. J. Artif. Organs 2022, 45, 919–926.
- El Rafei, A.; Trachtenberg, B.H.; Schultz, J.; John, R.; Estep, J.D.; Araujo-Gutierrez, R.; Suarez, T.E.; Goodwin, K.; Cogswell, R. Association between digoxin use and gas-trointestinal bleeding in contemporary continuous flow left ventricular assist device support. J. Heart Lung Transplant. 2021, 40, 671–676.
- Schettle, S.; Al Bawardy, B.; Asleh, R.; Sherazi, S.; Rajan, E.; Stulak, J.; Pereira, N. Danazol treatment of gastrointestinal bleeding in left ven-tricular assist device–supported patients. J. Heart Lung Transplant. 2018, 37, 1035–1037.
- Feng, N.; Chen, H.; Fu, S.; Bian, Z.; Lin, X.; Yang, L.; Gao, Y.; Fang, J.; Ge, Z. HIF-1α and HIF-2α induced angiogenesis in gastrointestinal vascular mal-formation and reversed by thalidomide. Sci. Rep. 2016, 6, 27280.
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085.
- Kenyon, B.M.; Browne, F.; D’Amato, R.J. Effects of Thalidomide and Related Metabolites in a Mouse Corneal Model of Neovascu-larization. Exp. Eye Res. 1997, 64, 971–978.
- Namdaran, P.; Zikos, T.A.; Pan, J.Y.; Banerjee, D. Thalidomide Use Reduces Risk of Refractory Gastrointestinal Bleeding in Patients with Continuous Flow Left Ventricular Assist Devices. ASAIO J. 2019, 66, 645–651.
- Ge, Z.; Chen, H.; Gao, Y.; Liu, W.; Xu, C.; Tan, H.; Chen, H.; Wei, W.; Fang, J.; Xiao, S. Efficacy of Thalidomide for Refractory Gastrointestinal Bleeding From Vascular Malformation. Gastroenterology 2011, 141, 1629–1637.e4.
- Seng, J.J.B.; Teo, L.L.; Chan, L.L.; Sim, D.K.; Kerk, K.L.; Soon, J.L.; Tan, T.E.; Sivathasan, C.; Lim, C.P. Novel Use of Low-dose Thalidomide in Refractory Gastrointestinal Bleeding in Left Ventricular Assist Device Patients. Int. J. Artif. Organs 2017, 40, 636–640.
- Draper, K.; Kale, P.; Martin, B.; Cordero, R.K.; Ha, R.; Banerjee, D. Thalidomide for treatment of gastrointestinal angiodysplasia in patients with left ventricular assist devices: Case series and treatment protocol. J. Heart Lung Transplant. 2015, 34, 132–134.
- Chan, L.L.; Lim, C.P.; Lim, C.H.; Tan, T.E.; Sim, D.; Sivathasan, C. Novel Use of Thalidomide in Recurrent Gastrointestinal Tract Bleeding in Patients with Left Ventricular Assist Devices: A Case Series. Heart Lung Circ. 2017, 26, 1101–1104.
- Hollis, I.B.; Chen, S.-L.; Chang, P.P.; Katz, J.N. Inhaled Desmopressin for Refractory Gastrointestinal Bleeding in a Patient With a HeartMate II Left Ventricular Assist Device. ASAIO J. 2017, 63, e47–e49.
More
