The photoconversion of CO2 into solar fuels seems to curb greenhouse effect and resolve the energy crisis. One of the challenges in developing practical CO2 photoconversion catalysts is to design materials with a low cost, high activity and good stability. The excellent photocatalysts based on TiO2, WO3, ZnO, Cu2O and CeO2 metal oxides that are cost-effective and long-lasting were discussed. Strategies to improve CO2 photoconversion efficiency are summarized and photocatalysts forms of 0D, 1D, 2D and 3DOM (zero/one/two-dimensional and three-dimensional-ordered macroporous, respectively) are involved, which can inspire the future improvement in photochemistry.
- photochemistry
- CO2 reduction
- metal oxide materials
- solar fuels
1. Introduction
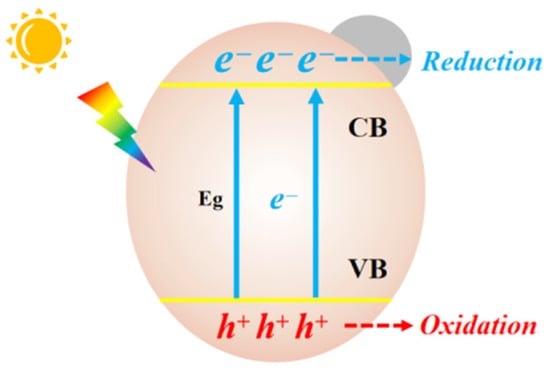
The transformation of CO2 can be driven by illumination, electricity and heat [1][3]. Solar energy is a safe, clean, renewable and inexhaustible energy source, so it is ingenious to achieve this conversion with sunlight [2][4]. Additionally, the reaction conditions in photocatalytic processes are mild [3][5]; thus, it is easy to conduct experimental tests. A new world opened up since H2 and O2 were obtained after radiating TiO2 with light. Extensive research about photocatalyst were conducted until the year of 2000. Since then, a growing number of materials have been designed and studied to absorb solar energy, including oxide semiconductors, sulfides (ZnS, CdS and MoS2), and polyoxometalates (Bi2WO6, Bi2MoO6 and BiFeO3) [4][5][6,7]. Organics, organometallic complexes, covalent organic polymers and noncovalent self-assembled supramolecular organic matter are also involved. Among them, inorganic metal oxide materials are widely studied for establishing efficient artificial photosystems due to their low cost, facile synthesis, stable crystal structures and environmental friendliness. TiO2, Cu2O, ZnO, WO3 and CeO2 show promising research value as the most common materials.
2. Theoretical Foundation and Strategies of Photocatalytic CO2 Reduction
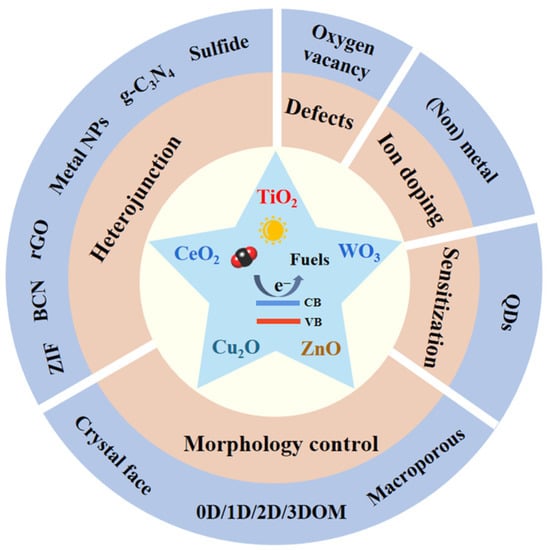
2.1. Morphological Control
2.1.1. Exposed Facet Adjustment
2.1.2. Quantum Dots (QDs)
2.1.3. One-Dimensional and Two-Dimensional Structures
One-dimensional nanostructured catalysts have high aspect ratios, such as nanowires, nanorods and nanotubes. The morphological tuning of the material makes a difference to their thermal, optical, electrical and magnetic properties [8][18]. For instance, TiO2 nanotube can act as a channel for electron transfer and build up the chemical reactions rate. Two-dimensional layered materials can protect a tiny particle component from aggregation. The CO2 adsorption capacity on 2D photocatalysts can be enhanced due to the large specific surface area and bountiful surface defects [9][19]. Two-dimensional lamellar nanosheets are widely used in photocatalysts, such as g-C3N4, MoS2 and WO3.2.1.4. Macroporous and Three-Dimensional Ordered Macroporous (3DOM) Structures
Macroporous materials are widely used in photocatalytic materials, owing to their excellent properties [10][20]. Unlike dispersed particles, sunlight can penetrate the pore wall easily and scatter widely inside the hollow structure, thus increasing the efficiency of illumination. Subsequently, the slender walls of pore reduce the transfer length of photo-generated charge carriers. Electrons (e−) and holes (h+) are separated more efficiently when heterojunctions are loaded on porous materials. The specific surface it provides is so large that more CO2 molecules have a chance to contact the catalyst for reduction reactions. Growing attention has been paid to hierarchical composite pores, including photonic crystal catalysis and separation of sub-microns. The slow light effect of photons associated with 3DOM materials have been considered to increase solar radiation absorption and enhance photocatalyst performance [11][21]. 3DOM products with periodic macrostructures [12][22], known as inverse opal, have been applied in battery materials, sensors, separation engineering and heterogeneous catalysis.2.1.5. Preparation of 3DOM Materials
Specifically, 3DOM materials are produced by the colloidal crystal template (CCT) method (Figure 34). Firstly, a uniform and close-connected organic sphere template can be obtained, through three key processes. Then, seeping metallic salt sol into the void of microspheres. After heat treatment, the organic microsphere template fades away and leaves a metal oxide frame. (i) The polymerizable monomer (methyl methacrylate, styrene) and initiator are mixed and heated under the protection of Ar; (ii) the earlier reaction liquid is filtered with microfiltration membrane; (iii) the microsphere mixture is centrifuged at a high speed for a long time, yielding the polymethylmethacrylate (PMMA) or polystyrene (PS) template; (iv) the template is immersed in the precursor solution and (v) is calcinated.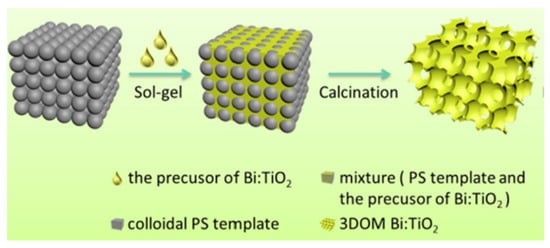
2.2. Heterojunction
2.3. Defects
2.4. Ion Doping
2.5. Sensitization
3. Photocatalysts with Different Basis Matrices
3.1. TiO
2
-Based Photocatalysts
Titanium dioxide (TiO2) is notable in photochemistry, with advantages such as non-toxic, cheapness, corrosion resistant, good physical and chemical stability. However, owing to the wide Eg of 3.0–3.2 eV, TiO2 only absorbs energy in the ultraviolet region (3–5% of the solar energy), and photoexcited charge pairs are easy to combine, resulting in quantum inefficiency. Usually, TiO2 is divided into the rutile, anatase and brookite on the basis of atomic arrangement modes. Rutile TiO2 is thermodynamically stable and does not distort or decompose at high temperatures. It has a narrower energy gap (3.0 eV) and a wider spectral response than the anatase phase (3.2 eV). Rutile TiO2 seeds generally grow larger in size and tend to form an agglomerated structure. Smaller anatase TiO2 particles have a wider lattice gap and abundant surface oxygen defects, which make it favorable for ion doping and photoreactions.3.1.1. Morphological Control
In hollow nanotube-shaped catalysts, the transport speed of CO2 and photoproducts can be facilitated. Ru phase is inclined to form methane in CO2 hydrogenation process [22][36] and Yang et al. [23][37] entrapped Ru nanoparticles in TiO2 nanotubes. Restricted Ru nanoparticles were resistant to sintering and leaching in the Ru-in/TNT catalyst channel (Figure 45). Electrons tend to gather in the tubes because of a confinement effect, which leads to an abundant, accessible metallic phase. It is easier for high-priced Ru species to combine with free electrons and then exhibit a superb CH4 and CH3OH yield.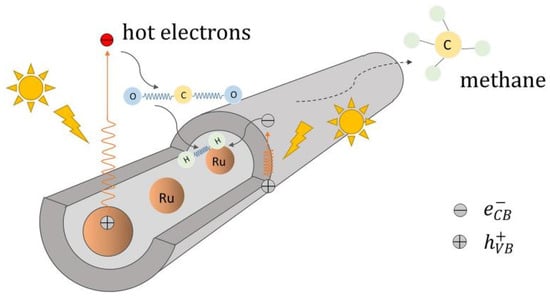
Exposed Facet Adjustment
A Pt-TiO2 single atomic site catalyst (PtSA/Def-s-TiO2) was prepared [25][41] by the “thermal solvent-argon treatment and hydrogen reduction” method. In order to construct Ti–Pt–Ti structures, TiO2 nanosheets with oxygen deficient sites were used to anchor monatomic Pt particles, which can retain the stability of isolated single atomic Pt and improve photocatalytic performance. The exposed (101) and (001) crystalline of TiO2 nanosheets were determined by transmission electron microscopy, and a thickness of 6.9 nm was observed through atomic force microscope. The EPR spectra of the samples confirm that the rich oxygen defect structure can be obtained by heating TiO2 nanosheets in argon atmosphere.3DOM Structure Ti-Based Materials
A ternary 3DOM Bi-doped TiO2 photocatalyst decorated with carbon dots (CDs) was obtained, whose pore engineering of the 3DOM skeleton greatly promoted the response in the whole solar spectrum range [13][26]. It exhibits enhanced photocatalytic performance because of its excellent exquisite structure and high charge transfer efficiency. Similarly, a BiVO4/3DOM TiO2 nanocomposite [26][43] was synthesized as a highly efficient photocatalytic catalyst for the degradation of dye pollutants.3.1.2. Heterojunction
p–n heterojunction: A p–n heterojunction is formed by combining p-type and n-type semiconductors. Even without light irradiation, electrons can diffuse from an n-type semiconductor to a nearby p-type one, in the case of the combination of two materials. Correspondingly, the holes on the surface of a p-type semiconductor are transferred to the n-type one, which results in an efficient separation of charge carriers. A ZnFe2O4-modified TiO2 was synthesized by the hydrothermal method [27][51], and the p-n heterojunction system could reduce CO2 to methanol at a yield of 75.34 μmol g−1 h−1. rGO composite: graphene materials have been widely used because of their large specific surface area, unique thermal stability and excellent electrical conductivity. Graphite nanomaterials are visible-light-responsive materials with appropriate band gaps, and the energy levels of CB and VB are in optimal positions relative to ordinary hydrogen electrodes. These unique photocatalytic properties have made them prime candidates for photocatalytic CO2 reduction. Fortunately, tightly contacted ultra-thin graphene layers and TiO2 compounds and can be prepared with some additives [28][52].3.1.3. Ion Doping
In recent years, elements such as B, N, Co and Bi have been widely applied in TiO2 doping. A carbon-based hybrid nanocomposite reduced graphene oxide (rGO), belonging to the narrow band gap, with oxygen-containing functional groups on the surface that can be enhanced by π interactions [29][57]. Laminar graphene carriers not only prevent TiO2 repolymerization, but also hybridize the function of the catalytic system. Co-doped TiO2 was loaded on the rGO [30][58], and the Co peak in EDX spectra and C-O peak in FT-IR spectra confirmed the successful doping and the presence of graphene support, respectively. The size of TiO2 particles decreased from 48–80 nm to 23–28 nm, which is consistent with earlier reports of changes in titanium doping with transition metal ions.3.1.4. Sensitization
A growing number of semiconductor materials are used to modify TiO2, but randomly mixed catalysts are not stable enough to achieve reproducibility. Therefore, Lee [31][59] grew well dispersed p-type NiS nanoparticles on the surface of a highly aligned n-type TiO2 film to obtain the NiS-sensitized TiO2 films. The band gaps of two components were estimated by wavelength relation. Some inferences can be drawn when considering the results of both the ultraviolet and visible spectra. It indicates that more electrons are subpoenaed from the short-Eg NiS and transferred to TiO2 conduction band. The spectra results reconfirmed the electron contribution of the NiS and the design of a catalyst that produced 3.77-fold CH4 compared to the TiO2 film.3.2. WO
3
-Based Photocatalysts
Tungsten-based oxides (WO3) have been extensively studied in recent decades and various morphologies have been presented. In the WO3 structure, the crystal in the stoichiometric ratio is connected with a twisted WO6 octahedra to form a perovskite crystal structure. It has monoclinic, orthorhombic and hexagonal crystal forms. At the same time, the oxygen lattice can be lost easily, resulting in oxygen vacancies and coordinatively unsaturated W atoms. Therefore, tungsten oxide has many non-stoichiometric compounds, such as WO2.72, WO2.8, WO2.83 and WO2.9.3.2.1 Morphological Control
Bi2WO6 is one of the tungsten-based materials that belongs to Aurivillius crystal oxides. Its crystal has an orthorhombic system, and its narrow band gap (2.7–2.9 eV) structure allows it to meet the response absorption of visible light. Moreover, its stable structure and eco-friendly properties have attracted many scientific researchers to study it. Since the valence band of Bi2WO6 is composed of O2p and Bi6p, and the conduction band is composed of W5d-assisted Bi6p orbitals, the VB energy levels can be dispersed broadly. By employing the Kirkendall effect in ion exchange and BiOBr precursor, Huang et al. [32][65] prepared a bowl-shaped Bi2WO6 HMS material. Based on the large specific surface area of the material, its adsorption capacity for CO2 reaches 12.7 mg g−1 at room temperature and pressure. The material adsorbs a large number of HCO3− and CO32− species on the surface during the reaction, which makes the catalytic reaction easier. The Bi2WO6 HMS thus has a high catalytic activity, and the methanol yield is 25 times higher than that of the Bi2WO6 SSR. Iron phthalocyanine FePc is neatly assembled on porous WO3 under induction and coupled with surface atoms by H-bonding [33][66]. The optimized FePc/porous WO3 nanocomposites exhibit enhanced CO2 photoreduction activity, which is attributed to the synergistic effects of a high specific surface area, a better charge separation and proper central metal cation. A series of mesoporous WO3 with interconnected networks were synthesized by the silica KIT-6 hard template method [34][67], which became oxygen-deficient after hydrogenation treatment. Both the ordered porous structure and oxygen vacancies contributed to the increased yield of CH4 and CH3OH. WO3 with a hollow nest morphology with hierarchical micro/nanostructures (HNWMs) was synthesized [35][68] by the one-step hydrothermal method (Figure 510), with a particle diameter of about 2.5 μm. The 2D nanosheets, which have an average thickness of 30–40 nm, were assembled to build a distinctive hollow nest structure with a good stability and reusability under visible light. Hao et al. [36][69] prepared core–shell heterojunctions of two-dimensional lamellar WO3/CuWO4 by the in situ method. After the modification of amorphous Co-Pi co-catalyst, the photoanode of ternary homogeneous core–shell structure exhibited a high photocurrent of 1.4 mA/cm2 at 1.23 V/RHE, which was 6.67 and 1.75 times higher than that of the pristine WO3 and 2D homogeneous heterojunction.
Preferentially Exposed Facets
3DOM Structure W-Based Materials
Unexpectedly, it was found that the resistance of 3DOM-WO3(270) and the Ag3PO4 electron absorption band were comparable. By depositing Ag3PO4 nanoparticles in the micropores of 3DOM-WO3, Chang et al. [41][79] achieved a higher photocatalytic activity and more efficient light harvesting at the wavelengths of 460–550 nm. A Z-scheme g-C3N4/3DOM-WO3 catalyst designed by Tang et al. [42][80] also has a high CO2 photoreduction activity.3.2.2. Heterojunction
Quantum dot composite: CuO quantum dots (QDs) were combined with WO3 nanosheets by a self-assembly method and the diameter of 6%CuO QDs/WO3 NSs was mainly located at 1.6 nm [43][81]. The bandgap energy of CuO/WO3 fell in 2.28 eV and the complex catalyst possessed a lower resistance for charge carrier transfer that showed in UV-vis DRS and EIS analysis. Due to the low CB position, CO cannot be obtained when using pure WO3. However, the photogenerated electrons gathered in the WO3 CB position was able to reach the CuO VB position when the Z-scheme (Figure 611) was formed by intimate heterojunctions. At the same time, the reduction reaction that transformed CO2 into CO occurred at the CuO CB position.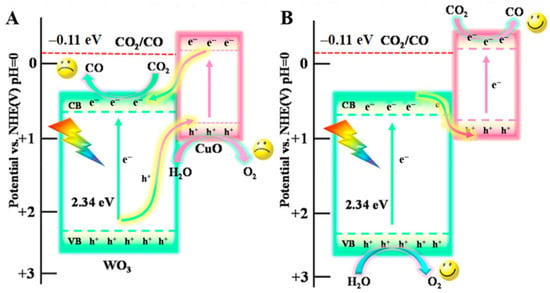
3.2.3. Ion Doping
3.3. ZnO-Based Photocatalysts
ZnO, a common metal oxide, is a n-type semiconductor with an Eg value of 3.37 eV. It is a kind of amphoteric oxide that has the advantages of nontoxic harmlessness, low cost, abundant reserves, convenient preparation, low dielectric constant and low optical coupling rate. ZnO has three main lattice structures: wurtzite structure, zinc-blended structure and tetragonal rock salt structure. The wurtzite structure is considered the most stable and common structure in nature. It is a kind of hexagonal crystal, in which the O and Zn atoms are aligned with the hexagonal density stacking.3.3.1. Morphological Control
The 3nm Pt particles were uniformly dispersed over ZnS@ZnO with a mesoporous heterostructure [44][90] and more CH3OH was obtained. Reactant charge carriers entered the pore channels of the porous heterozygous layer, thus reducing the likelihood of flow resistance and electron–hole recombination. The S-scheme photocatalyst delivered a high CH3OH formation rate of 81.1 μmol g−1 h−1, which is roughly 40 and 20 times larger than that of bare ZnO (3.72 μmol g−1 h−1) and ZnO–ZnS (4.15 μmol g−1 h−1). On the other hand, a porous ZnO@ZnSe core/shell nanosheet array material (Figure 712A) was prepared in a controlled manner [45][91]. The final n-type semiconductor composites had a proper negative CB band edge. In comparison to ZnO or ZnSe, more pairs of electron–holes were formed under visible light. Electrons tend to land on ZnO, which is aimed at methanol production. Mei et al. prepared a ZnO microsphere with different numbers of shells [46][92] and the photoelectric performance of ZnO was optimal when the number of shells reached three.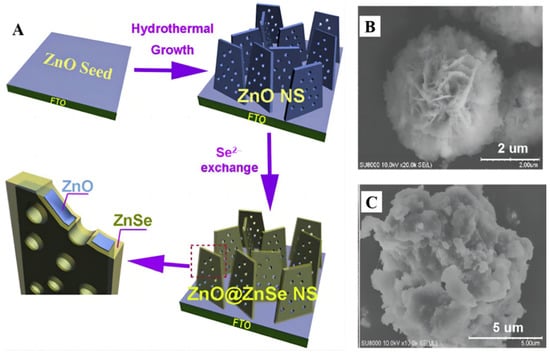
3DOM Structure Zn-Based Materials
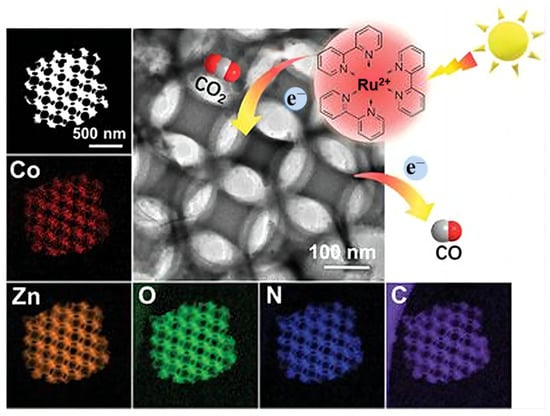
3.3.2. Heterojunction
3.4. Cu
2
O-Based Photocatalysts
Cuprous oxide (Cu2O) is a potential p-type semiconductor with a wide visible-light response range and high photo-electric conversion efficiency (18%) [50][106], and it displays attractive prospects in solar energy conversion and heterogeneous photocatalysis. Although Cu2O possesses many excellent properties, photocorrosion and the rapid recombination of e−/h+ pairs affect its activity and limit its application. The photocorrosion is believed to occur in two ways: (1) self-reduction caused by generated electrons and (2) self-oxidation caused by the generated holes.3.4.1. Morphological Control
A branch-like CdxZn1-xSe nanostructure was obtained [51][107] by the cation-exchange method, which was then mixed with Cu2O@Cu to form heterojunctions. Selenium (Se) vacancies were created during the ion exchange process and the crystal growth was limited due to the additive diethylenetriamine (DETA), leading to insufficient coordination of the surface atoms, which then become active adsorption sites. Highly hierarchical branching-like structures assembled by one-dimensional structural materials not only facilitate electron accumulation at their tips but also increase the light-accepting area, and characterization results show that branching structures can effectively absorb visible light. Cd0.7Zn0.3Se/Cu2O@Cu step-scheme heterojunction exhibited a CO release yield of 50.5 μmol g−1 h−1. Ultrafine cuprous oxide U-Cu2O (<3 nm) was grown on the polymeric carbon nitride (PCN) (Figure 915) by the in situ method [52][108]. PCN has a narrow band gap of 2.7 eV that can capture visible light. Both ultrafine nanoclusters and Z-scheme heterojunction can protect U-Cu2O from degradation. The photocatalyst U-Cu2O-LTH@PCN has high stability, maintaining more than 95% activity after five cycles of testing, while bare Cu2O grades completely within three cycles. A large number of heterojunctions were formed by U-Cu2O particles and lamellar PCN, expediting the electron transfer efficiency. The product can convert CO2 to methanol with water vapor under light irradiation at the high yield of 73.46 μmol g−1 h−1.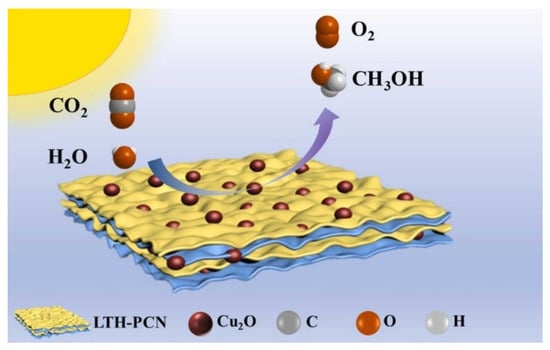
Preferentially Exposed Facets
3DOM Structure Cu-Based Materials
3DOM Structure Cu-Based Materials
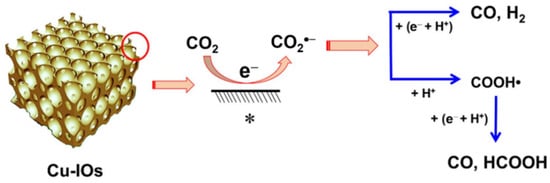
3.4.2. Heterojunction
3.5. CeO
2
-Based Photocatalysts
Cerium oxide (CeO2) has an octahedral face-centered cubic fluorite structure, in which the coordination numbers of Ce and O are 8 and 4. When reduced at a high temperature, it can be converted to nonstoichiometric CeO2−x (0 < x < 0.5). Notably, CeO2−x maintains a fluorite crystal structure and forms oxygen vacancies after losing a certain amount of oxygen. CeO2−X materials with different Ce/O ratios were also obtained in different conditions and it could be reconverted to CeO2 again if it returned to an oxidizing environment. Because of the unique electrical structure, cerium oxide (CeO2) is famous for the conversion sates between Ce4+ and Ce3+, which have been studied as oxygen storage catalytic materials and solid oxide full cells by many scholars [58][59][124,125]. In summary, CeO2 is a rare-earth metal oxide with a good photochemical stability, low cost and environment friendly characteristics.3.5.1. Morphology Control
Yb-, Er-doped CeO2 hollow nanotubes were synthesized [60][127] using silver nanowires coated with silica, and the products had a narrower band gap of 2.8 eV. The core–shell structured CeO2 was converted into mesoporous hollow spheres by the Ostwald ripening method in the presence of urea and hydrogen peroxide [61][128]. CeO2 nanocages can be fabricated by mixing (NH4)2Ce(NO3)2 with templates of Cu2O nanocubes [62][129], in which Cu2O is finally sacrificed. The photocatalytic results [63][130] indicated that CeO2 nanocages exhibit higher activity than hollow spheres.Preferentially Exposed Facets
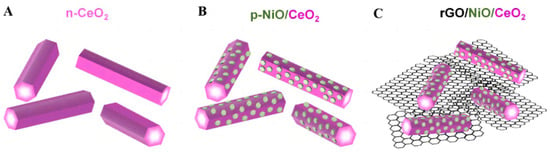
It was found that molecular CO2 can be distorted and participate in reactions at a low energy on the CeO2 surface [64][131]. A p-type NiO material was designed to modify the rod-like CeO2 nanostructure [65][132], allowing electrons and holes to migrate to opposite directions. They then operated the Mott–Schottky test, which showed a typical p–n junction. The presence of hexagon-shaped NiO plates broadened the range of light responses, which can be verified in the UV-Vis absorption spectra. Graphene oxide (rGO) was introduced as a “network” of for photoreduction electron transportation (Figure 129A–C). The impedance can be seen in the EIS Nyquist plot, which shows that the NiO/CeO2/rGO achieved the minimum value. The HCHO production rate of the ultimate catalyst was 421 μmol g−1h−1 with the synergy of several favorable factors. It is worth mentioning that a range of in situ techniques have been used to detect oxygen vacancies, structural changes, free radicals and formate on the surface of CeO2.