Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 4 by Jessie Wu.
“Drop-in” fuels, which can be blended with standard jet fuels without any modification to the current technology in any aspect and also extended to the automotive sector, would ensure a fast implementation into the fuel market. Until today, only six alternative fuels for aircraft have been accepted for blending with jet fuel and mainly biodiesel, bioethanol, and Hydrogenated Vegetable Oils for automotive fuel. In the case of aircraft fuel, some examples of incorporation of Fatty Acid Methyl Esters (FAME) blended with jet fuel are known, but over 5 mg/kg of FAME cannot be used for specific technical reasons. The decarboxylation of fatty acids has produced potential aircraft biofuels, but these have poor cold flow behavior. For fuels derived from the petrochemical industry, aromatic and other cyclic hydrocarbons efficiently increase the fuel density with little loss of gravimetric energy density. However, most alternative fuels have to be blended with their respective standard fuel in order to meet the density requirements. In addition to jet fuels, specialty military fuels have even more demanding density requirements, including the gravimetric net heating value, freezing and flash point, and storage stability, among others. It is essential to evaluate the feedstock sources, extraction, conversion, quality, and final use of biofuels in order to achieve new sustainable and industrially feasible biofuels. One of the most promising feedstocks is terpenic waste.
- terpenes
- terpenoids
- biofuels
- catalyst
1. Processes for Valorization of Terpenic Feedstock as Fuels
Terpenes and terpenoids have an extensive use in industry as sources of fine chemicals, flavors, polymers, fragrances, etc. According to their chemical structure, they are suitable for catalytic conversion into fuels or fuel additives.
The chemical transformation of most of the terpene feedstocks is possible, versatile, and not new. This is an advantage to find cheap, effective, and efficient routes to upgrade or to transform basic terpenes into fine chemicals, especially to convert them into potentially useful biofuels. The use of a heterogeneous solid catalyst for this purpose has also been well reported for several decades. An extensive review on the main transformation and upgrade of basic terpenes is reported by Swift [1]. A wide range and nature of solid catalysts can be used to transform basic terpenes using hydrogenation, isomerization, or other organic synthesis paths. An overview of the main solid catalysts reported for the conversion of terpenes is presented in Table 1, which is based on information collected, structured, and organized from a comprehensive report on this subject. As can be observed, most of the reports included in Table 1 show that researchers have used raw material for the synthesis based on commercial products and not the natural source, and many works do not include the assessment of catalyst reuse, meaning that the subject of study is not the catalyst performance and that the studies are not focused on reducing the cost of the process.
Table 1. Transformation of terpenes using solid catalysts.
| Process | Terpenes | Source | Solid Catalysts | Yield (%) | Reuse Cycles | References |
|---|---|---|---|---|---|---|
| Hydrogenation | Myrcene | Pine resin | Pd-charcoal/Ni-Raney | nr | nr | [1][2] |
| Limonene | Millipore | Ni-Raney/Pd/PtO2 | 89.4 | nr | [2][3] | |
| Terpinolene | nr | Ni-Raney | nr | nr | [1] | |
| α-pinene | Millipore | Amberlyst-15/Ni-Raney/molecular sieve | 85.8 | nr | [3][4] | |
| β-pinene | Pine resin | Ni-Raney | nr | nr | [1][2] | |
| Camphene | Pine resin | Ni-Raney | nr | nr | [1][2] | |
| Verbenone | nr | NiO-MgO/Sn-Pt/PtSiO2 | nr | nr | [1][5] | |
| Geraniol | Aldrich | Pt/PtO2/Ni/MoS3/Rh-based/Ru-based | 98 | nr | [1][6] | |
| Citral | nr | Pd/MoS3/Ni/Cr-based/Pt-SiO2 | 80–92 | nr | [1][7] | |
| Nerol | Fluka | SiO2/Pt/SiO2/Pt/H-Y | 22–66 | nr | [8] | |
| Linalool | nr | Amberlyst 15/Pd-C | 81 | nr | [9] | |
| Isomerization | α-pinene | nr | Ankalite KT-3/TiO2/kaolin/natural zeolite/HCl-activated Montmorillonite/Al2O3/cation-exchanged bentonite/Ag-NiY zeolite/H3PW12O40-SiO2;TiO2;ZrO2·nH2O/Cs2.5H0.5PW12O40-Nb2O5;ZrO2;TiO2 | 35.9–80 | nr | [1][10][11][12] |
| β-pinene | nr | HCl-activated Montmorillonite/TA-4/MA-4 | 35.9–83 | nr | [1] | |
| Dipentene | nr | MoS3/γ�-Al2O3 | nr | nr | [1] | |
| Limonene | nr | Ti-SBA-15 | 0–33 | nr | [13] | |
| Dimerization | α-pinene | nr | Al-MCM-41 | 43–87 | 4 | [14] |
| Addition of alcohols | β-pinene | Sigma | H3PW12O40·12·H2O | nr | 3 | [15] |
| Hydration | β-caryophyllene | copaiba oil | PW/PW-SiO2 | 70 | nr | [10] |
| dihydromyrcene | nr | PW/PW-SiO2 | nr | nr | [10] | |
| α-pinene | Sigma Turpentine |
HPW12O40/trichloroacetic acid-SiO2;TiO2;SiO2;Al2O3/Amberlyst 15/Y-Zeolite | 10.2–35.5 | 3–8 | [3][16][17][18][19] | |
| Oxyfunctionalization | β-pinene | GMP | Amberlyst 15 | nr | nr | [20] |
| Acetoxylation | β-caryophyllene | copaiba oil | PW/SiO2 | 70 | nr | [10][21] |
| α-terpineol | terpenic alcohols | AIPW12O40 | 92–95 | nr | [10] | |
| Alkylation | Limonene | nr | Amberlite IR120 | nr | nr | [1] |
| Camphene | nr | PW/SiW-TiO2/SiW-ZrO2 | nr | nr | [10] |
nr: no reported.
Among different chemical process, hydrogenation, isomerization, dimerization, alkylation, oxyfunctionalization, hydration, addition of alcohols, and acetoxylation are the most attractive. Part of the life cycle of the production and use of biofuels from terpenes is shown in Figure 1.
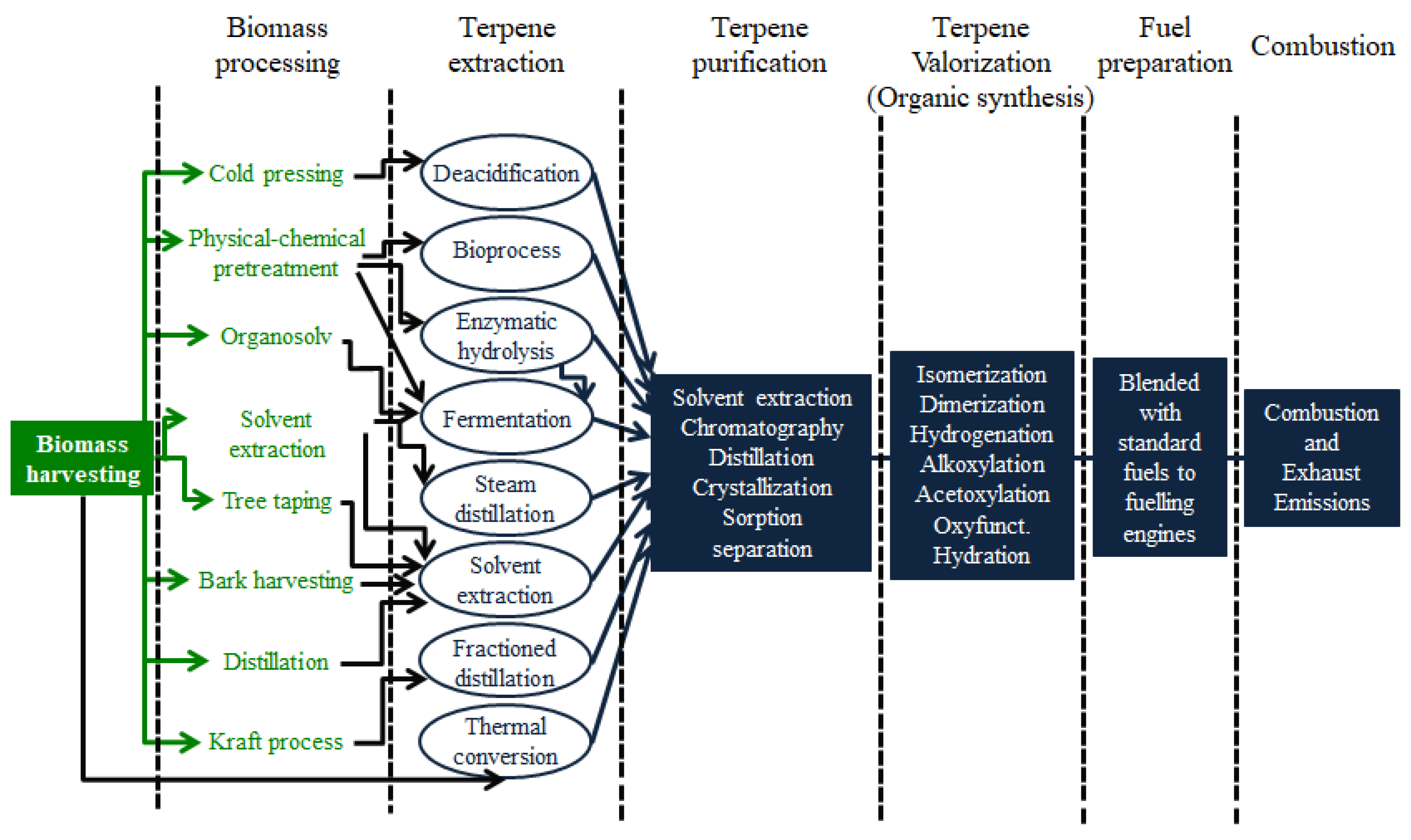
Figure 1. From well-to-wheel terpene processes, technologies, and pathways.
Hydrogenation is a well-known chemical process in which double bonds react with Hydrogen to reduce or eliminate the unsaturation in an organic compound. For several applications, particularly actual aircraft engines, double bonds and oxygen are not acceptable since they significantly affect energy density and fuel stability, and in this respect, hydrogenation of terpenes plays an important role. The hydrogenation process of α-pinene to cis-pinane is shown in Figure 2 beside other chemical conversion processes. Terpenes must be hydrogenated to saturated hydrocarbons before they can be used as fuels [22]. On the other hand, its high sooting tendency makes turpentine inadequate as a diesel fuel. Hydrogenation and oxyfunctionalization are the main processes used to tackle this drawback.
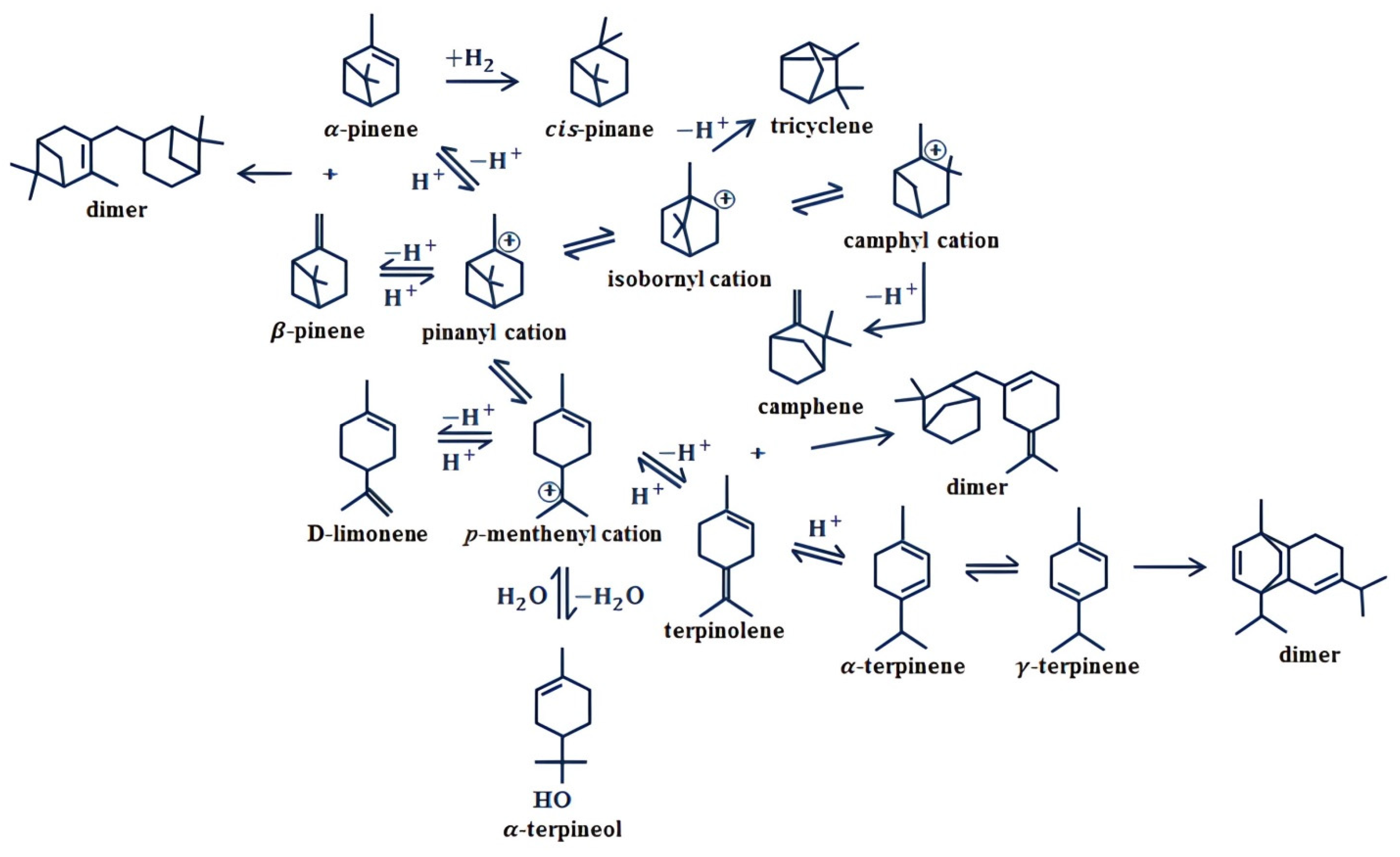
Figure 2. Reaction pathway scheme for the transformation of α-pinene and β-pinene based on acid catalysis.
Oxyfuncionalization is a type of chemical process in which Hydrogen is retired from a double bond structure and an oxygen group is introduced, changing the functional group of the terpene to ketones, aldehydes, esters, and ethers. This process is effective in changing characteristics of the terpene for its use as fuel or for other applications. For instance, oxyfunctionalized turpentine decreases particulate matter emissions, despite reducing its energy density [20], and low flash point can also be tackled using terpene oxyfunctionalization [23].
The dimerization process with an acid catalyst is effective in synthesizing high-energy density fuels from turpentine [24]. The acid-catalyzed dimerization of α-pinene is accompanied by isomerization, which is followed by cross-dimerization, making the reaction mixture consistent in isomers of α-pinene and dimers. To develop saturated hydrocarbon fuels from terpenes, the biosynthetic route is an attractive way, wherein organisms may convert cellulose- and/or hemicellulose-derived sugar solutions into neat terpene products [25]. According to several authors [26][27][28], the biosynthetic way is the closest approach to a sustainable production of terpenes. On the other hand, the chemical approach based on the use of heterogeneous solid catalysts from natural sources or industrial wastes is another approach to find sustainable routes for the production of drop-in fuels.
Isomerization of terpenes improves their cold flow properties for their use as fuel components. On the other hand, alkoxylation is an effective process for enhancing low-temperature properties, such as cloud and pour points. Oxyfunctionalization helps to reduce the sooting tendency of turpentine by converting unsaturated hydrocarbons and cyclic compounds. Hydration is a chemical route to obtain α-terpineol from α-pinene, leading to a higher octane number, leading to potential improvements in brake thermal efficiency and reduction of HC and CO exhaust emissions.
2. Production and Commercialization of Terpenes: Challenges for Their Use as Fuels
In 2019, 316,000 t of turpentine was produced around the world, of which 125,000 t was obtained from pine resin, 185,000 t from the paper industry, and only 6000 t from stump wood. China is the largest producer of turpentine in the world, being responsible for half of the global production, followed by Brazil. Other countries with important production levels are Indonesia, the United States of America, Portugal, Spain, Vietnam, Argentina, Mexico, and India [2]. There are more than one hundred countries around the world planting Eucalyptus, covering over 20 million hectares, making it the most widely planted broad-leaved tree species worldwide. More than 90% of the components of eucalyptus oil consists of 1,8-cineole [29]. As a fuel precursor, 1,8-cineole is converted to p-menthane, which is in the aircraft range of fuels. Nevertheless, the conversion process involves a series of reactions which frequently bring poor selectivity to p-menthane [30]. Commercially, terpenes have several industrial uses as agrochemicals, fragrances, nutraceuticals, and pharmaceuticals [31]. Terpenes are generally extracted using steam distillation. These extracts and steam distillates, also known as essential oils, are widely used to create perfumes, to refine flavors and aromas demanded by several industries, and for medicine. Terpenes and their derivatives represent a USD 650 million market globally [32]. They can also be manufactured from petrochemical sources and from terpene feedstocks. For instance, isoprene can be obtained through oil cracking, or as a by-product of naphtha. They are also extracted in small amounts from natural sources. The disadvantage of these processes is related to the production cost and the negative environmental impact generated by them. Due to the actual and future demand of terpenes, more economic and eco-friendly processes and related technologies are needed. Nonetheless, these methods must use inexpensive and non-toxic feedstocks. In this respect, microbial fermentation is a clear alternative for terpene production. Even so, not all bacteria can produce terpenes and/or their precursors as metabolic products. The application of biotechnology and genetic engineering to plants may considerably increase the yield of terpenes extracted, leading to a more attractive pathway and to further commercialization of products. Increases in more than 78% in monoterpene content after metabolic engineering is reported [33][34]. In recent years, biotechnology researchers have been reaching considerable advances in DNA and RNA sequencing, proteomics, and modification/editing tools such as CRISPR. According to Mewalal et al. [35], this is a favorable platform for the commercial scaling of terpenes in the near future. In the past, commercial-scale production of specific terpenes in plants was limited by relatively low yield, but today, some commercial-scale recovery of terpenes is established in biomasses such as pine, eucalypts, mints, and citrus. During the first ten years of growth, pine trees with an average of 4% wood terpene content may produce 40 GJ/ha/year of green energy [35]. From the same feedstocks, Wu et al. [36] estimate 206 GJ/ha/year. At 500 kg/ha/year, terpene production should find industrial uses in the energy sector. 20 million ha may produce 10 million tons of high-energy jet fuel [35], which could be used as standard fuel additives. Due to the wide range of sources of terpenes, there are important differences concerning production capabilities, cost of extraction, isolation, purification, and conversion, beside different levels of environmental impact and energy demand, especially for the distillation processes. On the other hand, sources are tackled by other feasibility constraints and concerns such as land availability. In this respect, most of the mentioned constrains are common for synthetically or biomass-produced terpenes. Researchers have explored the production of such compounds from biomass pyrolysis, but profitability in a short or medium term must be attended to. For example, the addition of down-stream steps focused on the extraction of terpenes could lead to more sustainable and profitable activity [37]. Despite the potential economic and environmental benefits of terpenes from biomass, several challenges must be overcome. Different species show different terpene content profiles, even influenced by stress and seasonal changes for the same strains [35]. A detailed proposal roadmap presented by Mewalal et al. [38] for the commercialization of specific terpenes proposes a system–biology approach through different plant organs. Nevertheless, how to overcome the scalability of the proposed road is not fully described. According to the authors, the development of short-rotation genotypes has the potential to reach high yields in the short and medium term. Intensive silvicultural management could also play an important role in maintaining and increasing the productivity of selected plants. Silviculture considerations may also include plantation sites, planting density, crop rotation (harvesting), fertilizer requirements, and pest management. For terpene production, in the case of Eucalyptus, shorter rotations will be advantageous, and 3000–5000 trees/ha are needed to boost productivity [39]. For commercial production, key factors include the levels of production and efficiency. In this respect, microbes may help to meet goals. For instance, bacteria would increase production of bisabolene to commercial levels, but even when it is similar to diesel fuel, the key to making it feasible economically requires production at high yields. Nevertheless, for the advance in industrial scale biofuel production from terpenes by applying biotechnology, they should be produced in microbes with a similar metabolic efficiency as ethanol, which is still not possible today. Amyris, a publicly traded company in Brazil, has produced terpene-based diesel-fueled buses in service for more than 5 million miles, and their terpene-based aircraft fuels have also been used. Their SIP-SPK jet fuel starts off as the terpene farnesene, produced directly from a microbe, and this is an interesting example showing the potential of production and use of biofuels made from terpenes. It is estimated that bisabolene could be produced at a final cost of 1.76 USD/kg. Currently, turpentine is commercialized in the market at around 1.3 EUR/L, but a decrease in the near future is expected. For pine resin with a productivity of 4 kg/pine/y, the biofuel’s cost would be 0.54 EUR/kg, and increasing the productivity to 6 kg/pine/y could reduce the production cost to 0.44 EUR/kg, which could be competitive with fossil fuels [23]. The challenge is to compete with the price of petroleum fuels and to develop new catalysts with high catalytic activity, selectivity, and operation at low temperatures in a short reaction time [40]. Plant extraction consumes large amounts of natural resources due to the low terpene content, and the downstream separation of similar terpenes in the extracts converts the separation into a complex process, limiting the extraction efficiency. In this respect, microbial conversion has the potential to tackle most of these drawbacks, but the microbial synthesis of terpenes is dependent on pathways based on microorganisms with low tolerance to toxicity. Due to this, the finding of effective microorganisms and the development of multilevel engineering strategies to increase biofuel’s yield is a main developmental line. On the other hand, CRISPR technology may represent another way to reach industrial-scale production of terpenes. Metal-based catalysts are still an insufficiently explored technology to cover the actual scale-up production of terpenes and their upgrading. Most of the solid catalysts reported and used are based on metals and zeolites. Nevertheless, zeolite’s tuning and metal doping (by nanotechnologies) are a vast field of development targeted to find cheaper and more effective catalysts. In this respect, the use of self-industry waste as precursors for preparation of solid catalysts is not well studied.References
- Swift, K.A. Catalytic transformations of the major terpene feedstocks. Top. Catal. 2004, 27, 143–155.
- Donoso, D.; Ballesteros, R.; Bolonio, D.; García-Martínez, M.J.; Lapuerta, M.; Canoira, L. Hydrogenated Turpentine: A Biobased Component for Jet Fuel. Energy Fuels 2021, 35, 1465–1475.
- Woodroffe, J.; Harvey, B.G. High-performance, biobased, jet fuel blends containing hydrogenated monoterpenes and synthetic paraffinic kerosenes. Energy Fuels 2020, 34, 5929–5937.
- Yu, F.; Xie, L.; Wu, F.; Yuan, B.; Xie, C.; Yu, S.; Liu, X.; Wang, L.; Wang, D. Mild Hydrogenation of α-Pinene Catalyzed by Ru Nanoparticles Loaded on Boron-doped Amphiphilic Core-Shell Mesoporous Molecular Sieves. ChemCatChem 2019, 11, 1518–1525.
- Casella, M.L.; Santori, G.F.; Moglioni, A.; Vetere, V.; Ruggera, J.F.; Iglesias, G.M.; Ferretti, O.A. Stereoselective hydrogenation of terpenes using platinum-based catalysts. Appl. Catal. A-Gen. 2007, 318, 1–8.
- Bernas, H.; Bernas, A.; Mäki-Arvela, P.; Leino, R.; Murzin, D.Y. Hydrogenation of geraniol using ruthenium-BINAP catalysts. Catal. Sci. Technol. 2012, 2, 1901–1907.
- Huang, Y.; Qiu, S.; Xu, J.; Lian, H. Hydrogenation of Citral to Citronellal Catalyzed by Waste Fluid Catalytic Cracking Catalyst Supported Nickel. ACS Omega 2020, 6, 476–482.
- Mäki-Arvela, P.; Kumar, N.; Paseka, I.; Salmi, T.; Murzin, D.Y. Support effects in nerol hydrogenation over Pt/SiO2, Pt/H-Y and Pt/H-MCM-41 catalysts. Catal. Lett. 2004, 98, 173–179.
- Keller, C.L.; Doppalapudi, K.R.; Woodroffe, J.D.; Harvey, B.G. Solvent-free dehydration, cyclization, and hydrogenation of linalool with a dual heterogeneous catalyst system to generate a high-performance sustainable aviation fuel. Commun. Chem. 2022, 5, 113.
- Gusevskaya, E.V. Reactions of terpenes catalyzed by heteropoly compounds: Valorization of biorenewables. ChemCatChem 2014, 6, 1506–1515.
- Alsalme, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. α-Pinene isomerisation over heteropoly acid catalysts in the gas-phase. Appl. Catal. A-Gen. 2010, 390, 219–224.
- Comelli, N.A.; Grzona, L.; Masini, O.; Ponzi, E.N.; Ponzi, M.I. Obtention of camphene with H3PW12O40 catalysts supported on TiO2, SiO2 and ZrO2nH2O. J. Chil. Chem. Soc. 2004, 49, 245–250.
- Retajczyk, M.; Wróblewska, A. The isomerization of limonene over the Ti-SBA-15 catalyst-the influence of reaction time, temperature, and catalyst content. Catalysts 2017, 7, 273.
- Zou, J.J.; Chang, N.; Zhang, X.; Wang, L. Isomerization and dimerization of pinene using Al-incorporated MCM-41 mesoporous materials. ChemCatChem 2012, 4, 1289–1297.
- Polo, H.P.; Lopes, N.P.G.; da Silva, M.J. Exploring the Keggin-Type Heteropolyacid-Catalyzed Reaction Pathways of the ?-Pinene with Alkyl Alcohols. Catal. Lett. 2019, 149, 2844–2853.
- Ávila, M.C.; Comelli, N.A.; Rodríguez-Castellón, E.; Jiménez-López, A.; Flores, R.C.; Ponzi, E.N.; Ponzi, M.I. Study of solid acid catalysis for the hydration of α-pinene. J. Mol. Catal. A Chem. 2010, 322, 106–112.
- Mochida, T.; Ohnishi, R.; Horita, N.; Kamiya, Y.; Okuhara, T. Hydration of α-pinene over hydrophobic zeolites in 1,4-dioxane-water and in water. Microporous Mesoporous Mater. 2007, 101, 176–183.
- Wijayati, N.; Pranowo, H.D.; Jumina, J.; Triyono, T. Syntheis of terpineol from α-pinene catalyzed by TCA/Y-Zeolite. Indones. J. Chem. 2011, 11, 234–237.
- Yang, G.; Liu, Y.; Zhou, Z.; Zhang, Z. Kinetic study of the direct hydration of turpentine. Chem. Eng. J. 2011, 168, 351–358.
- García, D.; Bustamante, F.; Villa, A.L.; Lapuerta, M.; Alarcón, E. Oxyfunctionalization of turpentine for fuel applications. Energy Fuel 2020, 34, 579–586.
- da Silva, K.A.; Rodrigues, N.V.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly acid catalysts in the valorization of the essential oils: Acetoxylation of β-caryophyllene. Appl. Catal A-Gen. 2010, 374, 87–94.
- Woodroffe, J.D.; Lupton, D.V.; Garrison, M.D.; Nagel, E.M.; Siirila, M.J.; Harvey, B.G. Synthesis and fuel properties of high-energy density cyclopropanated monoterpenes. Fuel Process Technol. 2021, 222, 106952.
- Donoso, D.; García, D.; Ballesteros, R.; Lapuerta, M.; Canoira, L. Hydrogenated or oxyfunctionalized turpentine: Options for automotive fuel components. RSC Adv. 2021, 11, 18342–18350.
- Cho, S.M.; Choi, J.H.; Kim, J.H.; Koo, B.; Choi, I.G. Catalytic Conversion of α-Pinene to High-Density Fuel Candidates Over Stannic Chloride Molten Salt Hydrates. Appl. Sci. 2020, 10, 7517.
- Meylemans, H.A.; Quintana, R.L.; Harvey, B.G. Efficient conversion of pure and mixed terpene feedstocks to high density fuels. Fuel 2012, 97, 560–568.
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483.
- Zargar, A.; Bailey, C.B.; Haushalter, R.W.; Eiben, C.B.; Katz, L.; Keasling, J.D. Leveraging microbial biosynthetic pathways for the generation of ‘drop-in’ biofuels. Curr. Opin. Biotechnol. 2017, 45, 156–163.
- Liu, C.L.; Tian, T.; Alonso-Gutierrez, J.; Garabedian, B.; Wang, S.; Baidoo, E.E.K.; Benites, V.; Chen, Y.; Petzold, C.J.; Adams, P.D.; et al. Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 2018, 11, 285.
- Hua, L.S.; Chen, L.W.; Antov, P.; Kristak, L.; Tahir, P.M. Engineering Wood Products from Eucalyptus spp. Adv. Mater. Sci. Eng. 2022.
- Yang, X.; Li, T.; Tang, K.; Zhou, X.; Lu, M.; Ounkham, W.L.; Spain, S.M.; Frost, B.J.; Lin, H. Highly efficient conversion of terpenoid biomass to jet-fuel range cycloalkanes in a biphasic tandem catalytic process. Green Chem 2017, 19, 3566–3573.
- Duong, L.H.; Reksowardojo, I.K.; Soerawidjaja, T.H.; Fujita, O.; Neonufa, G.F.; Nguyen, T.T.; Prakoso, T. Soap-derived biokerosene as an aviation alternative fuel: Production, composition, and properties of various blends with jet fuel. Chem. Eng. Process 2020, 153, 107980.
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240.
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for bio-based solvents created as petrochemical and fuel products transition towards renewable resources. Int. J. Mol. Sci. 2015, 16, 17101–17159.
- Lange, B.M.; Ahkami, A. Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes-current status and future opportunities. Plant Biotechnol. J. 2013, 11, 169–196.
- Guo, Z.; Yan, N.; Lapkin, A.A. Towards circular economy: Integration of bio-waste into chemical supply chain. Curr. Opin. Chem. Eng. 2019, 26, 148–156.
- Wu, H.; Fu, Q.; Giles, R.; Bartle, J. Production of mallee biomass in Western Australia: Energy balance analysis. Energy Fuels 2008, 22, 190–198.
- Joyce, B.L.; Stewart, C.N. Designing the perfect plant feedstock for biofuel production: Using the whole buffalo to diversify fuels and products. Biotechnol. Adv. 2012, 30, 1011–1022.
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Külheim, C.; Peter, G.F.; Tuskan, G.A. Plant-derived terpenes: A feedstock for specialty biofuels. Trends Biotechnol. 2017, 35, 227–240.
- Zalesny, R.S.; Cunningham, M.W.; Hall, R.B.; Mirck, J.; Rockwood, D.L.; Stanturf, J.; Volk, T.A. Woody biomass from short rotation energy crops. In Sustainable production of fuels, chemicals, and fibers from forest biomass. Am. Chem. Soc. 2011, 1067, 27–63.
- Miądlicki, P.; Wróblewska, A.; Kiełbasa, K.; Koren, Z.C.; Michalkiewicz, B. Sulfuric acid modified clinoptilolite as a solid green catalyst for solvent-free α-pinene isomerization process. Microporous Mesoporous Mater. 2021, 324, 111266.
More
