Primary progenitor tenocytes are diploid cells that may be cultured in vitro and therapeutically used for allogeneic musculoskeletal regenerative medicine. Firstly, technical aspects of cell banking, biotechnological manufacturing, and extensive preclinical characterization data have confirmed that FE002-Ten primary progenitor tenocytes may be safely considered for human cytotherapeutic use (e.g., in tissue engineering products, standardized transplants). Parallelly, lyophilized progenitor tenocyte extracts (e.g., stabilized cells or cell-free derivatives) were shown to optimally act as potent hyaluronan-based hydrogel functionalizing agents, useful for stability enhancement against oxidative product degradation. Therefore, primary progenitor tenocytes (e.g., FE002-Ten cell source) may potentially be used in diverse clinical presentations of tendon-related pathologies, ranging from volumetric tissue replacement (i.e., for the promotion of enhanced graft bio-integration) to local management of tissular inflammation and pain (i.e., ancillary action of the cellular extracts for the functional enhancement of injectable hyaluronan-based preparations). Overall, the primary progenitor tenocytes investigated under the Swiss progenitor cell transplantation program were shown to represent highly standardized biotechnological materials with a versatility of potential therapeutic uses after formulation into an array of cytotherapeutic preparations or cell-free devices.
1. Introduction
While numerous translational applications have been investigated for diverse tissue engineering and cytotherapeutic approaches, it is probable that cutaneous and musculoskeletal affections will be most rapidly addressed at large clinical scale
[1][2][3][1,2,3]. Notably, tendon tissue traumatic defects or degenerative affections are highly prevalent, yet few effective therapeutic interventions enable rapid and high-quality restoration of tissular structures and functions
[4][5][6][4,5,6]. Therein, injectable hyaluronan-based hydrogels have been successfully clinically used for mild to moderate tendinopathy cases, providing some anti-inflammatory properties, tissular lubrication or gliding enhancement, and local immunomodulatory properties
[7][8][9][7,8,9]. Biological-based regenerative approaches to tendon injuries and acute pathologies have notably comprised the injection use of bone marrow or adipose-derived stem cells, amniotic cells, placenta cells, tenocytes, tendon sheath fibroblasts, and platelet derivatives (e.g., platelet-rich plasma, PRP)
[10][11][12][13][14][15][16][10,11,12,13,14,15,16]. Therein, clinical outcomes and success of the intervention were reported to be highly dependent on the retained therapeutic indication and on the related treatment protocols
[11][17][18][19][11,17,18,19].
Primary progenitor tenocytes (i.e., FE002-Ten cell source) have been studied under the Swiss progenitor cell transplantation program as a potential ad hoc cytotherapeutic material source for optimal homologous and allogeneic management of tendinous tissue disorders
[20][21][22][20,21,22]. Firstly, this specific approach was technologically based on primary diploid cell biobanking concepts from the 1960s, which enabled the development of the global vaccine industry (e.g., WI-38, MRC-5 cell types)
[23][24][23,24]. Secondly, the renewed and optimized application of these early biotechnology concepts was enabled by modern technical advancements in the cell manufacturing and bioengineering fields, for enhanced process standardization and biological material quality
[21][25][21,25]. Based on these robust conceptual and technical elements, clinical-grade primary progenitor tenocytes (i.e., FE002-Ten cells) were procured and established as a cryopreserved cellular source for allogeneic musculoskeletal regenerative medicine applications under modern manufacturing quality requirements
[21].
The primary progenitor tenocytes investigated under the Swiss progenitor cell transplantation program (i.e., FE002-Ten cells) were shown to represent highly standardized biotechnological materials with high versatility in potential therapeutic uses
[20][21][22][23][26][27][20,21,22,23,26,27]. Primary progenitor tenocytes are cultured diploid cells (i.e., characterized by a finite in vitro lifespan during serial cell cultivation) that may be clinically used for allogeneic musculoskeletal regenerative medicine
[20][22][20,22]. Notably, primary progenitor cells are inherently pre-terminally differentiated, display pure and monomodal phenotypes in vitro, and are highly robust during serial monolayer cell expansion under consistent manufacturing parameters and technical specifications
[20][21][28][20,21,28]. Importantly, primary progenitor tenocytes are characterized by high biocompatibility, an inherent immune privilege, and by the absence of in vitro and in vivo tumorigenic behaviors
[22][26][22,26].
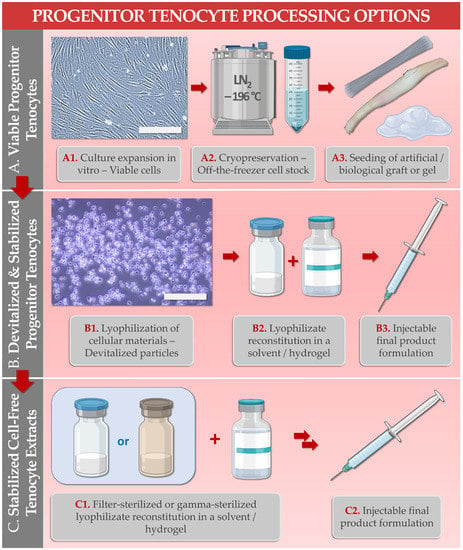
Following in vitro manufacturing under defined multi-tiered progenitor cell banking workflows, viable cellular lots may be used for off-the-freezer preparation of cell-seeded tendon allografts (i.e., using synthetic tendon/ligament scaffolds or decellularized biological tendon matrices) for tissue engineering (
Figure 1A)
[22][26][22,26].
Figure 1. Schematic and illustrated overview of the differential processing options for primary progenitor tenocytes (e.g., FE002-Ten cells), within the manufacture of cytotherapies or cytotherapy-inspired preparations for tendinous tissue disorders under the Swiss progenitor cell transplantation program. (A1–A3) Viable cell lots may be manufactured in vitro and may be further cryogenically stored for off-the-freezer preparation of tissue engineering products (i.e., using artificial or biological scaffolds) or injectable hydrogel-based (e.g., hyaluronan) preparations and devices. Scale bar = 200 µm. (B1–B3) Bulk cellular materials may be further stabilized by lyophilization for off-the-shelf availability and may be eventually reconstituted in hyaluronan-based injectable hydrogels. Scale bar = 400 µm. (C1,C2) Bulk cellular materials may also be further processed for fractionation and sterilization (i.e., 0.22 µm filtration or 60Co gamma irradiation) before storage or in view of final reconstitution in hyaluronan-based injectable hydrogels.
Alternatively, viable FE002-Ten tenogenic cells may be combined with hyaluronan-based hydrogels for the local delivery of cytotherapeutic payloads to tendon lesion sites in view of promoting or supporting tissular repair and/or regeneration (
Figure 1A)
[9]. In particular, various technical aspects of cell banking and extensive characterization data have confirmed that FE002-Ten primary progenitor tenocytes may be safely considered for human cytotherapeutic use
[21][22][21,22]. Furthermore, primary progenitor tenocytes may be used as biological starting materials for the standardized preparation of an array of stabilized cellular derivatives (
Figure 1B)
[27][29][27,29]. Specifically, it was shown that cytotherapy-inspired lyophilized preparations containing FE002 primary progenitor tenocyte derivatives or extracts present significant intrinsic antioxidant functions (e.g., Trolox equivalent antioxidant capacity, TEAC)
[29]. In detail, the lyophilized progenitor tenocyte extracts (e.g., stabilized cell-free fractions) were shown to act as potent hyaluronan-based hydrogel functionalizing agents for injectable product stability enhancement against oxidative degradation (
Figure 1C)
[27]. Such stabilized and sterilizable extracts may be used for the significant stability enhancement of various types of hyaluronan-based hydrogels, which may be clinically applied in mild to moderate cases of tendinopathy
[27].
Overall, the translational qualification of FE002-Ten cells, performed over the past decade in Switzerland, has confirmed the applicability of such standardized biological materials as cellular active ingredients or as starting materials within the development of therapeutic products and devices for human use
[22][29][22,29]. In detail, a growing compilation of peer-reviewed scientific reports has been constituting the multifaceted body of knowledge available around the FE002-Ten primary progenitor tenocyte source of interest (
Table 1).
Table 1. Descriptive listing of the various peer-reviewed international scientific publications describing the FE002-Ten primary progenitor tenocytes investigated under the Swiss progenitor cell transplantation program. Within this evolving body of knowledge, cultured primary progenitor FE002-Ten cells and derivatives were established as versatile therapeutic biological material contenders, notably in musculoskeletal regenerative medicine. CAM, chorioallantoic membrane model; GLP, good laboratory practices.
| Study Subject/Domain |
Scope of Study Data/Investigated Parameters |
References |
| 1. FE002-Ten Cell Source Establishment |
Establishment of the FE002-Ten cell source in a cryopreserved multi-tiered biobank following a single controlled organ donation. |
[21] |
| 2. FE002-Ten Cell Type In Vitro Characterization |
Characterization of primary progenitor tenocyte attributes (e.g., cell population homogeneity and purity, genetic and phenotypic stability, proteomic contents, biological functions) | 1 | . |
[9][20][21][22][28] | [9,20,21,22,28] |
| 3. FE002-Ten Cell Type Biobanking & Manufacturing |
Establishment of optimized and standardized in vitro primary progenitor tenocyte manufacturing workflows for the production of industrial scale cellular material lots. |
[22][28] | [22,28] |
| 4. FE002-Ten Cell Type Preclinical Safety Characterization |
Characterization of primary progenitor tenocyte safety (i.e., at clinically relevant passage levels | 2 | ) in vitro (e.g., genetic stability, tumorigenicity assays) and in vivo (e.g., CAM model, GLP study of cell implantation in rabbit tendons). |
[20][22] | [20,22] |
| 5. FE002-Ten Cell Type Derivative Manufacturing, Lyophilization, and Sterilization |
Establishment of biological material processing and purification workflows, for cell-derived and cell-free stabilized formulation obtention. Optimization of pharmaceutical processing (e.g., two-step lyophilization) for temperature stabilization of the cellular extracts. Optimization of the sterilization methodologies (e.g., submicron filtration, | 60 | Co gamma irradiation) for conservation of cell-derived extract critical quality attributes and functional properties. |
[27][29] | [27,29] |
| 6. FE002-Ten Cells or Derivatives: Study of Combination Product Prototypes |
Translational characterization of primary progenitor tenocytes for tissue engineering applications (e.g., using injectable hydrogels, collagen scaffolds, artificial and biological tendon matrices). Translational characterization of hyaluronan hydrogel-based devices incorporating stabilized cellular derivatives. |
[9][20]30] | [9,20 | [26][27][29][ | ,26,27,29,30] |
Therefore, primary progenitor tenocytes may potentially be used in diverse clinical presentations of tendon-related pathologies, ranging from volumetric tissue replacement (i.e., for the promotion of enhanced graft bio-integration) to the local management of tissular inflammation and pain (i.e., ancillary action of the cellular extracts for the enhancement of injectable hyaluronan-based preparations,
Figure 1)
[22][27][22,27]. Overall, primary progenitor FE002-Ten cellular materials were shown to be robust and well-adapted for processing in highly standardized manufacturing workflows, with a high versatility of potential therapeutic uses after formulation into a wide range of tissue engineering and biotechnological preparations (
Figure 1 and
Table 1)
[9][22][27][9,22,27].