- Aroma compounds
- Pyrus ussuriensis
- biosynthetic pathways
1. Fatty Acid Pathway
The primary aroma components of the ‘Nanguo’ pear are esters, which are biosynthesized by fatty acid metabolism (Figure 1) [1][17,38]. The β-oxidation of fatty acids is the primary biosynthetic process, which provides alcohols and acyl-CoA molecules to form esters [2][1]. Studies have found that the aroma volatiles in intact fruits are produced by β-oxidation. When plant tissue cells are destroyed, aroma volatiles are produced through the lipoxygenase (LOX) pathway [3]. However, some studies have shown that as the fruits mature, the membrane permeability increases, which increases the activity of the LOX pathway in the intact fruit. At this time, the LOX pathway can substitute for β-oxidation [4][3,46].
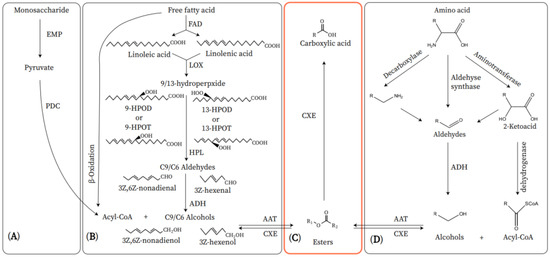
Figure 1. Metabolic pathway of volatile esters in fruit. (A) Monosaccharide pathway. (B) Fatty acid pathway. (C) CXE pathway. (D) Amino acid pathway. EMP: Embden-Meyerhof-Parnas; PDC: pyruvate dehydrogenase complex; FAD: fatty acid desaturase; LOX: lipoxygenase;9-HPOD: (10E,12Z)-9-hydroperoxy-10,12-oetadeeadienoic acid; 9-HPOT: (10E,12Z,15Z)-9-hydroperoxy-10,12,15-octadecatrienoic acid; 13-HPOD: (9Z,11E)-13-hydroperoxy-9,11-octadecadienoic acid, 13-HPOT: (9Z,11E,15Z)-13-hydroperoxy-9,11,15-octadecatrienoic acid; HPL: hydroperoxide lyase; ADH: alcohol dehydrogenase; AAT: alcohol acyl-CoA transferase; CXE: carboxylesterases.
In β-oxidation, acyl-CoA is reduced to aldehyde by acyl-CoA reductase, and the aldehyde is then reduced to alcohol by alcohol dehydrogenase (ADH) for alcohol acyltransferase (AAT) to produce esters [5][47]. The substrates of the LOX pathway are linolenic acid and linoleic acid, which can be obtained from free fatty acids under the action of fatty acid desaturase enzymes [6][37].
Linolenic acid and linoleic acid are derived through the LOX pathway into unsaturated short-chain alcohols, aldehydes, and esters [7][48]. Hydroperoxide lyase (HPL) is a downstream enzyme of LOX, which catalyzes the cleavage of hydroperoxide, the reaction product of LOX, into short-chain aldehydes [8][49]. Plant HPL is divided into two isozymes based on the difference of substrate peroxy group position. 13-HPL catalyzes the cleavage of the 13-position peroxy to produce C6 compounds, while 9-HPL cleaves the 9-position peroxy to form C9 compounds [9][50]. Next, alcohol dehydrogenase (ADH) catalyzes the interconversion of aldehydes and the corresponding alcohols. Finally, AAT catalyzes the reaction of acyl-CoA with alcohols to produce a variety of esters [5][47]. The alcohols involved in the reaction can be produced by the LOX pathway or reduced by short-chain acids produced by β-oxidation [2][1]. In addition, the LOX pathway can also produce jasmonic acid and its derivatives. In the allene oxide synthase (AOS) branch of the LOX pathway, 13-hydroxyperoxylinolenic acid is converted into 12,13-epoxyoctadecatrienoic acid through AOS, and jasmonic acid is then produced through a series of reactions. Jasmonic acid can be converted into the volatile ester methyl jasmonate by jasmonic acid carboxyl methyltransferase [10][51].
2. Amino Acid Pathway of Ester Biosynthesis
The amino acid metabolic pathway is also an important way to biosynthesize fruit aroma volatiles [11][52]. Aliphatic alcohols, aldehydes, and esters that contain branched chains can be biosynthesized through the amino acid metabolic pathway (Figure 1) [11][52]. A previous study found that the amino acids leucine, isoleucine, and valine could be the precursor of volatile alcohols, aldehydes, and esters in fruits, such as banana (Musa spp.), apple, strawberry (Fragaria × ananassa), and tomato (Solanum lycopersicum) [2,53,[12]54]. In strawberries, alanine can also serve as the precursor for volatile ethyl esters, which can be produced by AAT [12][54]. Amino acids are converted to the corresponding α-keto acids by aminotransferases, which are the key intermediates to convert amino acids into volatiles. α-Keto acids are then converted to volatile aldehydes or acyl-CoA in the substrate of α-keto acid decarboxylase or α-keto dehydrogenase. Subsequently, aldehyde and acyl-CoA are converted to esters by ADH and AAT [13][55].
3. Carbohydrate Pathway
Carbohydrates are not only the energy source of fruit metabolism but also an important source of fruit flavor, which can act as precursors for the biosynthesis of aroma volatiles (Figure 1) [14][17]. Carbohydrates can be decomposed into pyruvate by the Embden-Meyerhof-Parnas (EMP) pathway, and acetyl-CoA can be produced under the action of pyruvate dehydrogenase complex (PDC), which can be involved in the fatty acid pathway and contribute to the formation of esters [2][1]. Another pathway is that pyruvate forms acetaldehyde under the action of PDC, then acetaldehyde is reduced to ethanol under the catalysis of ADH, and then the ester is synthesized, but the pathway has not been confirmed.
Terpenoid Pathway
Terpenoids are biosynthesized from acetyl-CoA and pyruvate provided by carbohydrates in plastids and the cytoplasm. Although fatty acid oxidation is one of the primary pathways for the production of acetyl-CoA, this process may have little to do with the formation of terpenoids because fatty acid oxidation occurs in the peroxisome [2][1]. Terpenoids are the most abundant secondary metabolites, which are the primary aroma volatiles of citrus and grapes (Vitis vinifera) [15][56,57]. The terpenoids in the ‘Nanguo’ pear are primarily derived from α-farnesene [16][19]. α-Farnesene is a sesquiterpene-like volatile that can be biosynthesized via the mevalonate pathway [11][52]. The MVA pathway is carried out in the cytoplasm MEP pathway is in the plastid [11][52]. The biosynthetic precursors of terpenoids are isopentenyl pyrophosphate (IPP) and dimethylallyl diphosphate (DMAPP) [17][52,58]. Its biosynthesis has two pathways, which include the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway and the mevalonate pathway (MVA) (Figure 2) [11][52]. The products of the MEP pathway are monoterpenes and diterpenes, and the products of the MVA pathway are sesquiterpenes and triterpenes [17][58]. Acetyl-CoA is catalyzed by an enzyme to produce isopentenyl pyrophosphate (IPP). IPP is catalyzed by IPP isomerase to produce DMAPP, and it is then catalyzed by an enzyme to produce geranyl pyrophosphate (GPP) and farnesyl pyrophosphate (FPP) [11][52]. FPP is catalyzed by an enzyme to synthesize α-farnesene.
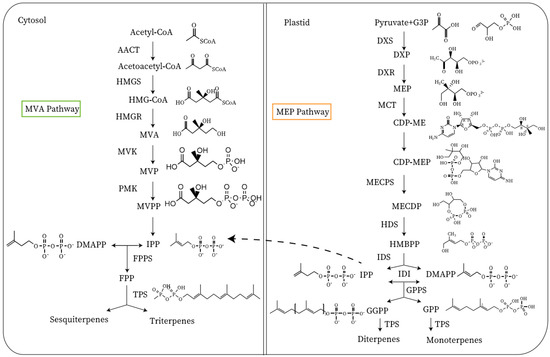
Figure 2. Synthesis of terpenoid volatile organic compounds. AACT, acetyl-CoA acetyltransferase; HMG-CoA, hydroxymethylglutaryl-CoA; HMGS, HMG-CoA synthase; HMGR, hydroxymethylglutaryl-CoA; MVA, mevalonic acid; MVK, mevalonate kinase; MVP, mevalonate 5-phosphate; PMK, phosphomevalonate kinase; MVPP, phosphomevalonate kinase; FPP, farnesyl pyrophosphate; FPPS, FPP synthase; G3P, glyceraldehyde 3-phosphate; DXS, DXP synthase; DXP, 1-deoxy-d-xylulose 5-phosphate; DXR, 1-deoxy-d-xylulose 5-phosphate reductoisomerase; MEP, methylerythritol phosphate; CDP-ME, 4-diphosphocytidyl-2-C-methyl-d-erythritol; CDP-MEP, CDP-ME 2-phosphate; MECDP, 2-C-methyl-d-erythritol 2,4-cyclodiphosphate; MECPS, MECPD synthase; HDS, 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase; HMBPP, (E)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate; IDS, isopentenyl diphosphate synthase; IPP, isopentenyl pyrophosphate; IDI, isopentenyl pyrophosphate isomerase; DMAPP, dimethylallyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; GGPPS: GGPP synthase; TPS: terpene synthase; GPP, geranyl pyrophosphate; GPPS, GPP synthase; MCT, 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase.
Moreover, the aroma of fruits is also regulated by carboxylesterases (CXE), which is an esterase that hydrolyzes esters [18][59]. Studies have found that the CXE in tomato and peach (Prunus persica) can use acetate as a substrate [19][20][60,61]. In pears, the content of CXE decreased with the extension of storage time, which could increase the accumulation of volatile esters in pears by reducing the degradation of esters [21][22][23][24][8].
