You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Mingshan Li and Version 2 by Lindsay Dong.
Drug and gene delivery systems mediated by nanoparticles have been widely studied for life science in the past decade. The application of nano-delivery systems can dramatically improve the stability and delivery efficiency of carried ingredients, overcoming the defects of administration routes in cancer therapy, and possibly maintaining the sustainability of agricultural systems. However, delivery of a drug or gene alone sometimes cannot achieve a satisfactory effect. The nanoparticle-mediated co-delivery system can load multiple drugs and genes simultaneously, and improve the effectiveness of each component, thus amplifying efficacy and exhibiting synergistic effects in cancer therapy and pest management.
- : cancer therapy
- co-delivery system
- nanoparticle
- nanopesticide
- RNA pesticide
1. Introduction
Over the past decade, nanotechnology has been at the forefront of rapid advances in fields as diverse as medicine, electronics, aerospace, life science, and agriculture [1][2]. The application of nanomaterials can break through the bottleneck of many traditional crafts and provide strong technical supports for nano-delivery platform, thus becoming a research hotspot in the fields of medicine and modern agriculture [3][4][5]. Since the first research on the delivery of drugs by nanomaterials, there have been numerous reports of the application of nanomaterials to deliver active ingredients (AIs) [6][7][8]. To date, many nanomaterials are employed for a nano-delivery system due to their unique physicochemical properties, such as controllable size, low cytotoxicity, enhanced activity of carried ingredients, and breaking the biofilm barrier. For example, polymeric NPs are fabricated from natural and synthetic polymers and are characterized by low cost and biodegradability [9]. Lipids are amphiphilic molecules consisting of a polar head group, a hydrophobic tail, and an intermediate linker [10]. Inorganic NPs are usually synthesized by chemical methods using heavy metal or inorganic material, such as mesoporous silica NPs [11][12], iron oxide NPs [13], gold NPs [14], and quantum dots, etc. [15]. Recently, plants or crops have also been used as feed stocks to develop green synthetic methods [16][17]. Multiple nanoparticles (NPs) have been designed and evaluated as carriers to deliver small molecule drugs for medical or agriculture field, including polymeric NPs, lipid NPs and other inorganic NPs [18][19].
Chemotherapy, biological therapy, and radiation therapy are the main forms of cancer treatment, and the former is also considered to be one of the most effective methods in clinical practice [20]. In chemotherapy, patients are often treated with cytotoxic drugs to kill cancer cells [21]. Biological therapy involves the application of biomacromolecules such as nucleic acids to inhibit specific molecules that affect tumor growth [22]. However, the use of chemotherapeutic agents is limited by three major limitations, such as poor water solubility, poor bioavailability, and toxicity of normal tissues [23]. Poor solubility and bioavailability often result in irregular biodistribution and systemic toxicity of chemotherapeutic drugs, which in turn affect normal cells. Multidrug resistance (MDR) caused by long-term and continuous administration is considered as a harmful consequence [24][25][26].
With nano-delivery platforms, small molecule drugs or nucleic acid molecules can be efficiently transported to target tissues without degradation [7][27]. However, single delivery of chemotherapy targeting one pathway is usually not enough, and multiple reasons (such as MDR) hinder the development of effective and long-lasting cancer treatments. Therefore, the combination of different treatments (delivery of genes or drugs) has been proposed as a more ideal cancer treatment strategy and widely studied [28][29][30]. Co-delivery systems can improve the pharmacokinetics and physicochemical properties of therapeutic drugs and improve the efficacy of combination therapy through targeted design of drug delivery regimens [31]. Many combination applications have been designed to achieve synergistic therapeutic effect, and the co-delivery of multiple AIs in the same nanocarrier may achieve desirable effects [32].
Pesticides play a vital role in defending against biological disasters and promoting crop productivity [33]. Traditional pesticides are synthetic organic compounds with high hydrophobicity, which is inconvenient to apply. Meanwhile, traditional processing and formulation requires organic solvents which further poses environmental pollution and biosafety risks [34][35]. Therefore, there is an urgency in scientific use of pesticides and improve the control efficacy of plant diseases and insect pests for green food production. Nanomaterials can be used as substitutes for organic solvents in processing and formulation. Currently, nano-enabled pesticides (nanopesticides) are considered to be less than 1000 nm in size, including insecticides, fungicides, herbicides, and rodenticides, as well as plant immune inducers, plant growth regulators and other AIs that can improve the resistance of plants [36][37]. For precision agriculture, nanopesticides are prepared in different formats of NPs, which show a variety of appealing characteristics, including long-term stability and duration, controlled and stimulation-regulated release rates, increased AI solubility, and improved adhesion to crops, etc. [38][39][40][41].
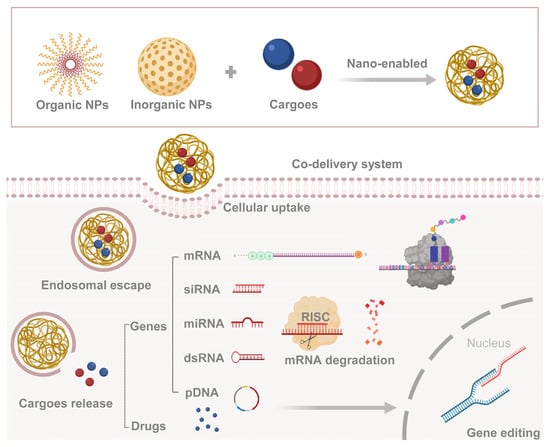
2. Co-Delivery System in Medical Field
Various NPs have been examined to design novel co-delivery systems, which can be divided into inorganic-based NPs and organic-based NPs. The former mostly includes mesoporous silica NPs, iron oxide NPs, metallic NPs (copper, gold, or silver), quantum dots, etc. The latter includes polymeric micelles, polymeric NPs, liposomes, dendrimers, etc. Recent advances in the development of NPs suggest that these systems can be designed to protect and deliver AIs with different types and sizes, ranging from chemical small molecules to biological macromolecules, and from hydrophilic to hydrophobic agents [32]. The drugs and/or genes (cargoes) are enabled by NPs for efficient cellular uptake and arrive at the target after the endosomal escape to take effect separately (Figure 1). In addition to many types of drugs, nucleic acid molecules come in many varieties, including messenger RNA (mRNA) which is decoded into peptides or proteins; microRNA (miRNA), short interfering RNA (siRNA), and double-stranded RNA (dsRNA) that can induce gene silencing; and plasmid DNA (pDNA) that gets further expression in the nucleus, etc.
Figure 1. Combination route and mechanism of co-delivery system. Cargoes (genes or drugs) are encapsulated in nanoparticles, and then delivered into the cytoplasm through endosomal escape.
2.1. Co-Delivery of Drugs
Based on the achievements obtained from the delivery of single chemical drug, co-delivery of two different chemical drugs has been developed and clinically applied to treat different types of cancers [42][43][44]. Compared with monotherapy, combination therapy can not only reduce the possibility of tumor resistance to drugs, but also alleviate the side effects of drugs by reducing the dose of drugs. Different NPs are designed for delivery because of the different physical, chemical and biological properties of these therapeutic agents. Current studies have shown that the delivery of two chemical drugs in the same nanocarrier is much more efficient than a system that delivers a single drug [43][45]. Meanwhile, nanocarriers can improve the water solubility and delivery efficiency of hydrophobic drugs in vivo.
On this basis, co-delivery of other chemotherapeutic drugs or natural active products also achieves synergistic therapeutic effect [46]. Chao and co-workers reported a mesoporous magnetite ferrite NP as an inorganic drug carrier, which can efficiently encapsulate hydrophobic drug (rifampin) and simultaneous co-load hydrophilic drug (isoniazide) [47]. Besides, the prepared NPs exhibit excellent biocompatibility and cellular uptake, which can enhance drug loading capacity and solve the delivery problem of hydrophobic drug molecules [48].
2.2. Co-Delivery of Genes
Nucleic acid-based gene therapy is based on therapeutic molecules DNA or RNA, which aims to achieve multiple goals in vivo, including (1) deliver siRNA, miRNA or dsRNA for gene down regulations; (2) deliver pDNA or mRNA for gene over expression [49][50]. Co-delivery of the nucleic acids has the potential to regulate target gene expression level, hence changing protein content and even disease development. Similar to co-delivery of antitumor drugs, different formulations containing various nucleic acid molecules have been screened for overcoming MDR [51]. In 2013, Tabernero et al. used lipid NPs to co-deliver two modified siRNAs and performed the first human clinical trials [52]. Ball et al. established the co-delivery system of siRNA and mRNA based on the same lipid NP that can enhance the efficacy of both agents in vitro and in vivo [53]. NPs co-delivering siRNA and mRNA can mediate significantly higher levels of gene silencing compared to NPs loading siRNA alone. When the same set of cells is assessed for mRNA delivery, the co-delivery system again produces better results. Yang et al. used nano-carriers to co-deliver K-ras and Notch siRNA [54]. This strategy increases the sensitivity of pancreatic cancer cells to the chemotherapy drug gemcitabine and also helps to resolve MDR. Wang et al. designed and constructed liposomal NPs loaded with both p38α MAPK and p65 siRNA [55].2.3. Co-Delivery of Genes and Drugs
Although many effective research studies and treatments have been made, nucleic acids face the same problems with cancer heterogeneity and adaptive resistance as traditional small molecule drugs in cancer therapy. With the achievements obtained from the fields of chemotherapy and gene therapy, co-delivery of drugs and genes has attracted wide attention in combination therapy due to its synergistic therapeutic effects [56][57][58]. The general incentive behind the co-delivery system is to disrupt MDR signaling pathways. For example, the combination of anticancer drugs and siRNA has great potential in cancer treatment to achieve synergistic effect and overcomes the hurdlers of using a single drug [59][60].3. Co-Delivery System in Agricultural Field
In agricultural and environmental fields, some nanoparticles can be used alone due to their own properties [61]. Metal oxides TiO2 have been shown to have excellent dye degradation activity and can be applied for environmental remediation [16][62]. Biosynthesized AuNPs modulated the accumulation of nitric oxide and induced salt stress tolerance in wheat plants [63]. Meanwhile, NPs can be directly used as nanopesticides due to their antibacterial or insecticidal properties [62].3.1. Nanoparticles Deliver Pesticides (Drugs)
Nanopesticides are similar to other common pesticide formulations in that they help to improve the apparent solubility of the insoluble AIs, or release the AIs in a slow or targeted manner, thereby protecting them from premature degradation [64][65][66]. For nanopesticides composition, AIs can be loaded on the inorganic NPs surface, incorporated into the pores of porous NPs or conjugated with polymer. The high surface-to-volume ratio of silica NPs has been widely used as nanofertilizers and nanopesticides [67][68]. Polymeric NPs are of significant interest for encapsulation of pesticides due to many unique features such as renewable, biodegradable, low cost, and environmental responsibility [69]. Yan et al. used a polymeric NP (Star polycation, SPc) to assemble with botanical pesticide matrine, reducing its particle size to 10 nm in aqueous solution and amplifying its bioactivity by about 20% in vitro and in vivo [70]. The SPc can not only increase the bioactivity of loaded pesticides, but also reduce pesticide residue [66][71]. The SPc can also assemble with calcium glycinate to prepare a calcium nutrition nanoagent with nanoscale size (17.72 nm), thus enhancing transport and antiviral immunity [72]. The calcium transport is accelerated into tomato leaves and the protective effect of calcium glycinate is remarkably improved toward tomato mosaic virus. NPs can greatly improve the environmental stability of AIs and build a controlled release system of agents that respond to external pH, enzyme, light, temperature, and other factors [73]. The stimulus-responsive nanocarriers typically employ widely available and biodegradable natural polymers including ethyl cellulose and starch. Liu et al. developed a composite that chemically functionalized chitosan and attapulgite clay as pesticide carriers capable of responding to UV-accelerated release [74].3.2. Nanoparticles Deliver Nucleic Pesticides (Genes)
RNA interference (RNAi) is a conserved regulatory mechanism mediated by the siRNA pathway, microRNA pathway, and Piwi-interacting RNA pathway, which can silence or inhibit the expression of target genes [75][76][77]. For nanopesticides, the addition of NPs enhances the stability of nucleic acid molecules and makes them free from degradation. The lipid formulation of dsRNA is protected from the degradation by endonucleases present in Sf9 cell conditioned medium, hemolymph, and mid-intestinal cavity contents of Spodoptera frugiperda [78]. For another example, SPc and perylenediimide-cored cationic dendrimer can prevent dsRNA from degradation by RNase A and hemolymph of aphids and fall armyworms [79]. In addition to shielding and protecting dsRNA from nuclease degradation in the environment, NPs can also facilitate the transport of dsRNA across the membrane and avoid its degradation in endosomes or lysosomes. For instance, a cationic core–shell fluorescent nanoparticle is able to accelerate endocytosis and deliver DNA across cell membrane for efficient cellular uptake [80]. Lu and co-workers designed the block copolymer poly to form well-defined, core–shell NPs to facilitate its passage through various physiological obstacles and thus prolong the survival time of dsRNA in the digestive tract, so as to enter the midgut cells of Locusta migratoria [81]. The SPc can also efficiently deliver dsRNA across the cell membrane and achieve efficient gene silencing [82]. Compared to naked dsRNA, crucial genes regulating endocytosis and exocytosis are remarkably up-regulated in Sf9 cells treated with a dsRNA/SPc complex [79]. RNAi-based strategy has great potential in combatting plant diseases and pests [83][84][85]. Crops can be directly sprayed with dsRNA (spray-induced gene silencing, SIGS) targeting key genes of plant pathogens or pests to induce specific silencing, thus leading to the decline of pest infestation and finally realizing the sustainable eco-friendly pest management [86][87]. A new formulation was developed with the help of a fluorescent NP. The RNA pesticide rapidly penetrates the insect body wall and effectively inhibits gene expression [88].3.3. Application of Co-Delivery System
Firstly, aour team constructed SPc as a low-cost multifunctional nanocarrier that can co-deliver the dsRNA and pesticide to develop a novel multicomponent nano-pesticide against devastating green peach aphids[89]. The SPc can self-assemble with botanical pesticide matrine, and then complex with dsRNA to form a nano-sized matrine/SPc/dsRNA complex, which can be efficiently delivered into Drosophila S2 cells. The dsRNA (dshem) targeting immune gene hemocytin leads to efficient gene silencing and a high mortality rate through SPc-based topical application, and the main lethal mechanism is via the down-regulating hem gene, resulting in severe bacterial infection. In the field trial, the dshem/SPc complex exhibits short persistence, and the matrine/SPc complex shows slow-acting property, exposing their defects. Interestingly, both initial acting time and persistence of co-delivery complex are remarkably improved, which overcomes the disadvantages of both agents. The synergistic effect of co-delivery system based on NPs has achieved good performance in pest control. The co-administration of thiamethoxam and dsRNA of synapsin, both targeting the nervous system, effectively results in the death of melon aphids [90].4. Perspectives in Pesticide
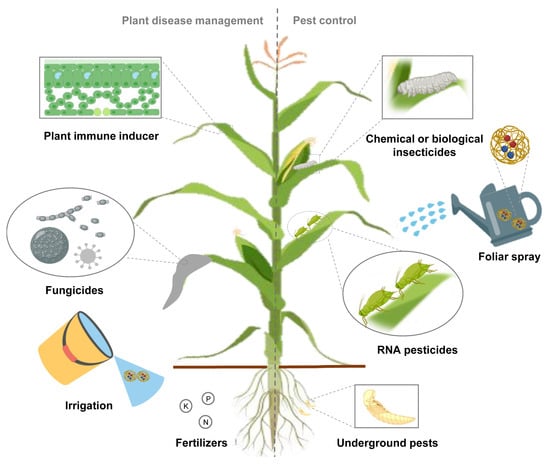
The application of NP-based co-delivery systems is mainly divided into synergistic and complementary functions. The co-delivery system, no matter delivering drugs, genes or multiple agents, should be based on solving the bottleneck of pesticide development. Using the synergistic mode of co-delivery system to concentrate on a certain direction, the corresponding drug and nanomaterials can be further reduced and enhanced [90]. For example, the use of co-delivery of conventional pesticides and their corresponding RNA pesticides targeting resistance-related genes avoids the high cost of developing new pesticides and gives traditional pesticides a new lease of life (unpublished data). On the other hand, complementary action in both aspects can reduce the frequency of pesticide application and the dosage of nanomaterials, which is friendlier to the environment [89]. To prevent or suppress plant diseases, researchers can develop nanofungicides for plant pathogens; immune inducers and multiple nanofertilizers for plant stress. A variety of insecticides, including chemical or biopesticides and RNA pesticides targeting pests, can be purposefully combined for both above and below ground pests (Figure 2). Multiple application methods including foliar spraying, irrigation, and trunk injection can also be refined to specific applications [86][91]. The production costs of NPs and RNA pesticides should be further reduced, and the application of co-delivery system in the field has been preliminarily realized.
Figure 2. Application of co-delivery system is promising in agricultural field. Fabrication of co-delivery nanopesticide system, assembled with insecticides, fungicides or fertilizers, achieves synergistic effects or multiple aspects of drug administration simultaneously.
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2020, 25, 112.
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnol. 2022, 20, 11.
- Wu, H.; Li, Z. Recent advances in nano-enabled agriculture for improving plant performance. Crop J. 2022, 10, 1–12.
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology strategies for plant genetic engineering. Adv. Mater. 2022, 34, 2106945.
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathol. Res. 2023, 5, 2.
- Roco, M.C.; Williams, R.S.; Alivisatos, P. Nanotechnology Research Directions: IWGN Workshop Report. Vision for Nanotechnology R&D in the Next Decade; Springer: Berlin/Heidelberg, Germany, 2000.
- Chen, F.; Liu, Q.; Xiong, Y.; Xu, L. Nucleic acid strategies for infectious disease treatments: The nanoparticle-based oral delivery route. Front. Pharmacol. 2022, 13, 984981.
- Yang, Z.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Chen, L.; Jin, Z. Fabrication of zein–carboxymethyl cellulose nanoparticles for co-delivery of quercetin and resveratrol. J. Food Eng. 2023, 341, 111322.
- Lai, P.; Daear, W.; Löbenberg, R.; Prenner, E.J. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly(d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf. B 2014, 118, 154–163.
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094.
- Kamegawa, R.; Naito, M.; Miyata, K. Functionalization of silica nanoparticles for nucleic acid delivery. Nano Res. 2018, 11, 5219–5239.
- Kim, M.H.; Na, H.K.; Kim, Y.K.; Ryoo, S.R.; Cho, H.S.; Lee, K.E.; Jeon, H.; Ryoo, R.; Min, D.H. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano 2011, 5, 3568–3576.
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible iron oxide nanoparticles for targeted cancer gene therapy: A review. Nanomaterials 2022, 12, 3323.
- Tunc, C.U.; Culha, M. Gold nanoparticles conjugated DNA-tile nanomaterials for simultaneous delivery of morpholino antisense oligonucleotides and doxorubicin. J. Drug Deliv. Sci. Technol. 2022, 74, 103546.
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Delivery Sci. Technol. 2021, 61, 102308.
- Ahmad, M.M.; Kotb, H.M.; Mushtaq, S.; Waheed-Ur-Rehman, M.; Maghanga, C.M.; Alam, M.W. Green synthesis of Mn + Cu bimetallic nanoparticles using Vinca rosea extract and their antioxidant, antibacterial, and catalytic activities. Crystals 2022, 12, 72.
- Pieła, A.; Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Duda, M.; Grzesiak, J.; Saeid, A.; Klimek-Ochab, M. Biogenic synthesis of silica nanoparticles from corn cobs husks. Dependence of the productivity on the method of raw material processing. Bioorganic Chem. 2020, 99, 103773.
- Mogheri, F.; Jokar, E.; Afshin, R.; Akbari, A.A.; Dadashpour, M.; Firouzi-amandi, A.; Serati-Nouri, H.; Zarghami, N. Co-delivery of metformin and silibinin in dual-drug loaded nanoparticles synergistically improves chemotherapy in human non-small cell lung cancer A549 cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102752.
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299.
- DeVita, V.T.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653.
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic cancer: A review. JAMA 2021, 326, 851–862.
- Volker, S. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419.
- Annovazzi, L.; Mellai, M.; Schiffer, D. Chemotherapeutic drugs: DNA damage and repair in glioblastoma. Cancers 2017, 9, 57.
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291.
- Waghray, D.; Zhang, Q. Inhibit or evade multidrug resistance P-glycoprotein in cancer treatment. J. Med. Chem. 2018, 61, 5108–5121.
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233.
- Mercan, D.A.; Niculescu, A.G.; Grumezescu, A.M. Nanoparticles for antimicrobial agents delivery—An up-to-date review. Int. J. Mol. Sci. 2022, 23, 13862.
- Zashikhina, N.; Gladnev, S.; Sharoyko, V.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E.; Tennikova, T. Synthesis and characterization of nanoparticle-based dexamethasone-polypeptide conjugates as potential intravitreal delivery systems. Int. J. Mol. Sci. 2023, 24, 3702.
- Busa, P.; Kankala, R.K.; Deng, J.-P.; Liu, C.-L.; Lee, C.-H. Conquering cancer multi-drug resistance using curcumin and cisplatin prodrug-encapsulated mesoporous silica nanoparticles for synergistic chemo- and photodynamic therapies. Nanomaterials 2022, 12, 3693.
- Muniyandi, P.; Palaninathan, V.; Hanajiri, T.; Maekawa, T. Direct cardiac epigenetic reprogramming through codelivery of 5′azacytidine and miR-133a nanoformulation. Int. J. Mol. Sci. 2022, 23, 15179.
- Nezhadi, S.; Dorkoosh, F. Co-delivery systems: Hope for clinical application? Drug Deliv. Transl. Res. 2022, 12, 1339–1354.
- Sahrayi, H.; Hosseini, E.; Karimifard, S.; Khayam, N.; Meybodi, S.M.; Amiri, S.; Bourbour, M.; Farasati Far, B.; Akbarzadeh, I.; Bhia, M.; et al. Co-Delivery of letrozole and cyclophosphamide via folic acid-decorated nanoniosomes for breast cancer therapy: Synergic effect, augmentation of cytotoxicity, and apoptosis gene expression. Pharmaceuticals 2022, 15, 6.
- Enserink, M.; Hines, P.; Vignieri, S.; Wigginton, N.; Yeston, J. The pesticide paradox. Science 2013, 341, 728–729.
- Kaur, R.; Mavi, G.K.; Raghav, S. Pesticides classification and its impact on environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897.
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112.
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867.
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235.
- Wais, U.; Jackson, A.W.; He, T.; Zhang, H. Nanoformulation and encapsulation approaches for poorly water-soluble drug nanoparticles. Nanoscale 2016, 8, 1746–1769.
- Mattos, B.D.; Tardy, B.L.; Magalhães, W.L.E.; Rojas, O.J. Controlled release for crop and wood protection: Recent progress toward sustainable and safe nanostructured biocidal systems. J. Control. Release 2017, 262, 139–150.
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22.
- Deka, B.; Babu, A.; Baruah, C.; Barthakur, M. Nanopesticides: A systematic review of their prospects with special reference to tea pest management. Front. Nutr. 2021, 8, 686131.
- Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles loaded with docetaxel and resveratrol as an advanced tool for cancer therapy. Biomedicines 2022, 10, 1187.
- Khafaji, M.; Zamani, M.; Vossoughi, M.; Zad, A.I. Doxorubicin/cisplatin-loaded superparamagnetic nanoparticles as a stimuli-responsive co-delivery system for chemo-photothermal therapy. Int. J. Nanomed. 2019, 14, 8769–8786.
- Nechaeva, A.; Artyukhov, A.; Luss, A.; Shtilman, M.; Gritskova, I.; Shulgin, A.; Motyakin, M.; Levina, I.; Krivoborodov, E.; Toropygin, I.; et al. Synthesis of amphiphilic copolymers of N-Vinyl-2-pyrrolidone and allyl glycidyl ether for co-delivery of doxorubicin and paclitaxel. Polymers 2022, 14, 1727.
- Alven, S.; Aderibigbe, B.A. Efficacy of polymer-based nanocarriers for co-delivery of curcumin and selected anticancer drugs. Nanomaterials 2020, 10, 1556.
- Ataide, J.A.; Coco, J.C.; dos Santos, É.M.; Beraldo-Araujo, V.; Silva, J.R.A.; de Castro, K.C.; Lopes, A.M.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P.; et al. Co-encapsulation of drugs for topical application—A review. Molecules 2023, 28, 1449.
- Chao, X.; Yu, S.; Liu, L.; Wu, X.; Dai, H. Magnetically targeted co-delivery of hydrophilic and hydrophobic drugs with hollow mesoporous ferrite nanoparticles. RSC Adv. 2018, 8, 15326.
- Chen, D.; Wang, G.; Song, W.; Zhang, Q. Novel CD44 receptor targeting multifunctional “nano-eggs” based on double pH-sensitive nanoparticles for co-delivery of curcumin and paclitaxel to cancer cells and cancer stem cells. J. Nanopart. Res. 2015, 17, 421.
- Ultimo, A.; Orzaez, M.; Santos-Martinez, M.J.; Martínez-Máñez, R.; Marcos, M.D.; Sancenón, F.; Ruiz-Hernández, E. High-capacity mesoporous silica nanocarriers of siRNA for applications in retinal delivery. Int. J. Mol. Sci. 2023, 24, 2753.
- Abashkin, V.; Pędziwiatr-Werbicka, E.; Horodecka, K.; Zhogla, V.; Ulashchik, E.; Shmanai, V.; Shcharbin, D.; Bryszewska, M. Silver nanoparticles modified by carbosilane dendrons and PEG as delivery vectors of small interfering RNA. Int. J. Mol. Sci. 2023, 24, 840.
- Zare, M.; Pemmada, R.; Madhavan, M.; Shailaja, A.; Ramakrishna, S.; Kandiyil, S.P.; Donahue, J.M.; Thomas, V. Encapsulation of miRNA and siRNA into nanomaterials for cancer therapeutics. Pharmaceutics 2022, 14, 1620.
- Tabernero, J.; Shapiro, J.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417.
- Ball, R.L.; Hajj, K.A.; Vizelman, J.; Bajaj, P.; Whitehead, K.A. Lipid nanoparticle formulations for enhanced co-delivery of siRNA and mRNA. Nano Lett. 2018, 18, 3814–3822.
- Yang, C.; Chan, K.K.; Lin, W.J.; Soehartono, A.M.; Lin, G.; Toh, H.; Yoon, H.S.; Chen, C.K.; Yong, K.T. Biodegradable nanocarriers for small interfering ribonucleic acid (siRNA) co-delivery strategy increase the chemosensitivity of pancreatic cancer cells to gemcitabine. Nano Res. 2017, 10, 3049–3067.
- Wang, Y.; Wu, Q.; Wang, J.; Li, L.; Sun, X.; Zhang, Z.; Zhang, L. Co-delivery of p38α MAPK and p65 siRNA by novel liposomal glomerulus-targeting nano carriers for effective immunoglobulin a nephropathy treatment. J. Control. Release 2020, 320, 457–468.
- Sriram, V.; Lee, J.Y. Calcium phosphate-polymeric nanoparticle system for co-delivery of microRNA-21 inhibitor and doxorubicin. Colloids Surf. B 2021, 208, 112061.
- Fischer, T.; Winter, I.; Drumm, R.; Schneider, M. Cylindrical microparticles composed of mesoporous silica nanoparticles for the targeted delivery of a small molecule and a macromolecular drug to the lungs: Exemplified with curcumin and siRNA. Pharmaceutics 2021, 13, 844.
- Tabatabaei, S.N.; Derbali, R.M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J.L.; Hardy, P. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J. Control. Release 2019, 298, 177–185.
- Zhang, R.; Wei, S.; Shao, L.; Tong, L.; Wu, Y. Imaging intracellular drug/siRNA co-delivery by self-sssembly cross-linked polyethylenimine with fluorescent core-shell silica nanoparticles. Polymers 2022, 14, 1813.
- Biswas, S.; Deshpande, P.P.; Navarro, G.; Dodwadkar, N.S.; Torchilin, V.P. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials 2013, 34, 1289–1301.
- Zhao, S.; Huang, W.; Wang, C.; Wang, Y.; Zhang, Y.; Ye, Z.; Zhang, J.; Deng, L.; Dong, A. Screening and matching amphiphilic cationic polymers for efficient antibiosis. Biomacromolecules 2020, 21, 5269–5281.
- Ahmad, M.M.; Mushtaq, S.; Al Qahtani, H.S.; Sedky, A.; Alam, M.W. Investigation of TiO2 nanoparticles synthesized by Sol-Gel method for effectual photodegradation, oxidation and reduction Reaction. Crystals 2021, 11, 1456.
- Wahid, I.; Rani, P.; Kumari, S.; Ahmad, R.; Hussain, S.J.; Alamri, S.; Tripathy, N.; Khan, M.R. Biosynthesized gold nanoparticles maintained nitrogen metabolism, nitric oxide synthesis, ions balance, and stabilizes the defense systems to improve salt stress tolerance in wheat. Chemosphere 2022, 287, 132142.
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002.
- Guleria, G.; Thakur, S.; Shandiiya, M.; Sharma, S.; Thakur, S.; Kalia, S. Nanotechnology for sustainable agro-food systems: The need and role of nanoparticles in protecting plants and improving crop productivity. Plant Physiol. Biochem. 2023, 194, 533–549.
- Jiang, Q.; Xie, Y.; Peng, M.; Wang, Z.; Li, T.; Yin, M.; Shen, J.; Yan, S. Nanocarrier-pesticide delivery system with promising benefits in a case of dinotefuran: Strikingly enhanced bioactivity and reduced pesticide residue. Environ. Sci. Nano 2022, 9, 988–999.
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90.
- Naaz, H.; Rawat, K.; Saffeullah, P.; Umar, S. Silica nanoparticles synthesis and applications in agriculture for plant fertilization and protection: A review. Environ. Chem. Lett. 2022, 21, 539–559.
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.M.; Zade, A.K.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497.
- Yan, S.; Hu, Q.; Li, J.; Chao, Z.; Cai, C.; Yin, M.; Du, X.; Shen, J. A star polycation acts as a drug nanocarrier to improve the toxicity and persistence of botanical pesticides. ACS Sustain. Chem. Eng. 2019, 7, 17406–17413.
- Yan, S.; Hu, Q.; Jiang, Q.; Chen, H.; Wei, J.; Yin, M.; Du, X.; Shen, J. Simple osthole/nanocarrier pesticide efficiently controls both pests and diseases fulfilling the need of green production of strawberry. ACS Appl. Mater. Interfaces 2021, 13, 36350–36360.
- Yan, S.; Hu, Q.; Wei, Y.; Jiang, Q.; Yin, M.; Dong, M.; Shen, J.; Du, X. Calcium nutrition nanoagent rescues tomatoes from mosaic virus disease by accelerating calcium transport and activating antiviral immunity. Front. Plant Sci. 2022, 13, 1092774.
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; Pereira, A.E.S.; Proenca, P.L.F.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100.
- Liu, B.; Chen, C.; Teng, G.; Tian, G.; Zhang, G.; Gao, Y.; Zhang, L.; Wu, Z.; Zhang, J. Chitosan- based organic/inorganic composite engineered for UV light-controlled smart pH-responsive pesticide through in situ photo-induced generation of acid. Pest Manag. Sci. 2022, 78, 2299–2308.
- Ray, P.; Sahu, D.; Aminedi, R.; Chandran, D. Concepts and considerations for enhancing RNAi efficiency in phytopathogenic fungi for RNAi-based crop protection using nanocarrier-mediated dsRNA delivery systems. Front. Fungal Bio. 2022, 3, 977502.
- Akbar, S.; Wei, Y.; Zhang, M.-Q. RNA Interference: Promising approach to combat plant viruses. Int. J. Mol. Sci. 2022, 23, 5312.
- Menezes, P.S.; Yan, Y.; Yang, Y.; Mitter, N.; Mahony, T.J.; Mody, K.T. RNAi-based biocontrol of pests to improve the productivity and welfare of livestock production. Appl. Biosci. 2022, 1, 229–243.
- Gurusamy, D.; Mogilicherla, K.; Shukla, J.N.; Palli, S.R. Lipids help double-stranded RNA in endosomal escape and improve RNA interference in the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21678.
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124.
- He, B.; Chu, Y.; Yin, M.; Müllen, K.; An, C.; Shen, J. Fluorescent nanoparticle delivered dsRNA toward genetic control of insect pests. Adv. Mater. 2013, 25, 4580–4584.
- Lu, Q.; Cui, H.; Li, W.; Liu, T.; Chen, Q.; Qing, Y. Synthetic nanoscale RNAi constructs as pesticides for the control of Locust migratoria. J. Agric. Food Chem. 2022, 70, 10762–10770.
- Zhang, Y.; Ma, Z.Z.; Zhou, H.; Chao, Z.J.; Yan, S.; Shen, J. Nanocarrier-delivered dsRNA suppresses wing development of green peach aphids. Insect Sci. 2022, 29, 669–682.
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a foliar spray: Efficiency and challenges to field applications. Int. J. Mol. Sci. 2022, 23, 6639.
- Kiselev, K.V.; Suprun, A.R.; Aleynova, O.A.; Ogneva, Z.V.; Dubrovina, A.S. Physiological conditions and dsRNA application approaches for exogenously induced RNA interference in arabidopsis thaliana. Plants 2021, 10, 264.
- Zotti, M.; Santos, E.A.D.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250.
- Majumdar, S.; Keller, A.A. Omics to address the opportunities and challenges of nanotechnology in agriculture. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2595–2636.
- Wang, M.; Jin, H. Spray-induced gene silencing: A powerful innovative strategy for crop protection. Trends Microbiol. 2017, 25, 4–6.
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999.
- Qu, X.; Wang, S.; Lin, G.; Li, M.; Shen, J.; Wang, D. The synergistic effect of thiamethoxam and synapsin dsRNA targets neurotransmission to induce mortality in Aphis gossypii. Int. J. Mol. Sci. 2022, 23, 9388.
- Li, M.; Ma, Z.; Peng, M.; Li, L.; Yin, M.; Yan, S.; Shen, J. A gene and drug co-delivery application helps to solve the short life disadvantage of RNA drug. Nano Today 2022, 43, 101452.
- Pandhi, S.; Mahato, D.K.; Kumar, A. Overview of green nanofabrication technologies for food quality and safety applications. Food Rev. Int. 2021, 37, 1–21.
More
