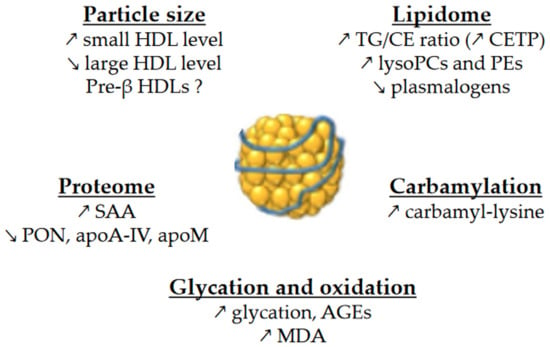
Glycation and glycoxidation may play a role in the accelerated catabolism of HDLs, although there is reportedly no correlation between HDL-apoA-I FCR and HbA
1c [35][96]. Thus, the turnover of glycated apoA-I is almost three times faster than its non-glycated counterpart
[36][97]. In addition, the methylglyoxal modification of HDLs reduces plasma half-life in vivo
[23][74]. Yet considerable evidence suggests that the kinetic properties of HDLs are linked to TG metabolism. For instance, the HDL-apoA-I FCR in obese individuals is associated with triglyceridemia
[38][39][99,100], VLDL-apoB PR
[38][99], VLDL
1-TG FCR and PR
[39][100], and also with the HDL-TG/CE ratio
[37][98]. In T2DM, the HDL-apoA-I FCR is also positively associated with plasma TG
[35][96] and with the TG content in HDLs
[14][35][44,96]. HDLs enriched in TGs are hydrolyzed by hepatic lipase, leading to lipid-poor HDLs. Such HDLs are thermodynamically unstable and exhibit structural modifications in apoA-I, which facilitates both its dissociation from HDL particles
[40][101] and its renal glomerular filtration
[41][102].
3. HDL Functions
3.1. Reverse Cholesterol Transport
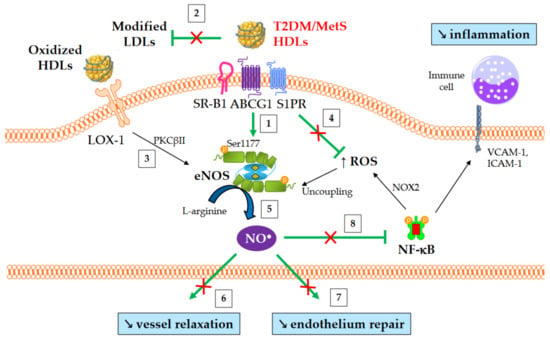
The best-known function of HDLs is their major role in RCT, which enables the removal of cholesterol from lipid-laden macrophages and artery walls. To sum up, HDLs promote cholesterol efflux from macrophage foam cells in atherosclerotic plaques either specifically by interacting with the transporters ABCA1 and ABCG1, or by aqueous diffusion, through a process facilitated by the scavenger receptor B1 (SR-B1). ABCA1 mediates cholesterol efflux preferentially to lipid-poor apoA-I and small dense HDLs, whereas ABCG1 transports cholesterol to the more mature lipidated HDL particles. Free cholesterol is then esterified by LCAT, and this esterification is important for maintaining the dynamics of cholesterol efflux. Cholesterol is ultimately cleared by the liver, either directly by selective uptake through SR-B1 or by a more recently discovered pathway mediated by F1-ATPase and P2Y13 receptor, or indirectly after CETP-mediated transfer to apoB-containing lipoproteins, which are then internalized by the LDL receptor. In addition to this classical hepatobiliary pathway, cholesterol can be also eliminated by a transintestinal pathway called transintestinal cholesterol efflux (TICE)
[42][105].
Cholesterol efflux is thus the first step in the atheroprotective RCT pathway, and the cholesterol efflux capacity (CEC) of HDL particles is a crucial determinant of cholesterol clearance from lipid-laden macrophages. Over the past few years, studies have demonstrated that the CEC of HDLs is more strongly and inversely associated with incident cardiovascular events than the circulating HDL-C level itself
[43][44][106,107].
Some authors reported an increased CEC in patients with T2DM
[45][27] and in diabetic patients with hypertriglyceridemia
[46][110]. ABCA1-dependent efflux was also increased using apoB-depleted serum from T2DM patients with hypertriglyceridemia compared to T2DM patients without hypertriglyceridemia
[47][111]. On the other hand, CEC in T2DM patients was unmodified using fibroblasts and whole plasma
[46][48][49][30,55,110], human THP-1 macrophages, and apoB-depleted serum
[11][41], or when using THP-1 cells and HDLs isolated by dextran sulfate precipitation
[28][81]. Lastly, CEC was decreased in T2DM patients using adipocytes and LpA-I (i.e., HDL particles containing apoA-I but not apoA-II)
[50][112], Fu5AH hepatoma cells and whole plasma/serum
[19][51][52][53][54][55][34,36,37,68,113,114], mouse peritoneal macrophages and isolated HDL3
[56][115], murine RAW264.7 macrophages and apoB-depleted serum
[36][97], and also THP-1 macrophages and isolated HDLs
[1][57][18,116]. Similarly, it was recently reported that small HDL particles and apoB-depleted serum from patients with T2DM both have impaired ABCA1-dependent CEC using baby hamster kidney (BHK) cells
[36][58][97,117]. However, medium and large HDL particles had a similar capacity to promote ABCA1-specific CEC in T2DM patients compared to control individuals in the study
[58][117]. Otherwise, all three sizes of HDL particles from T2DM subjects had similar ABCG1-dependent CEC compared to controls
[58][117].
Changes in lipid composition may also affect HDL CEC. In particular, the replacement of CE by TG molecules in HDLs affects the conformation of apoA-I
[15][45] and could therefore modulate binding to receptors. In addition, the literature suggests that the content of HDLs in total PLs
[59][60][134,135] and in SMs
[61][136] modulates CEC, but, as mentioned above, the changes in these parameters are very heterogeneous across studies. Cardner et al. recently reported that CEC of apoB-depleted serum is mainly driven by apoA-I level in diabetic individuals
[5][22].
3.2. Anti-Inflammatory Properties
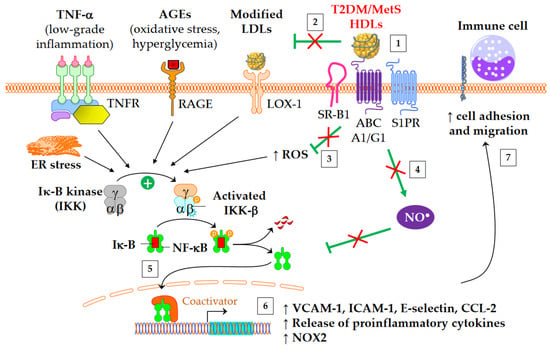
Both diabetes and obesity are associated with low-grade inflammation, which substantially contributes to endothelial dysfunction and atherosclerosis. As shown in
Figure 23, HDL particles exert an anti-inflammatory function by downregulating the expression of molecules involved in the recruitment of immune cells into the subendothelial space. These molecules include chemokine CCL-2
[62][137], vascular cell adhesion molecule (VCAM)-1, intracellular adhesion molecule (ICAM)-1, and selectin-E
[11][41]. In addition, HDLs inhibit the release of inflammatory cytokines, such as TNF-α and IL-1β. The HDL anti-inflammatory function seems of particular relevance for CV outcomes, since it predicts new cardiac events in patients with myocardial infarction, independently of HDL-C
[63][138]. Moreover, an inverse association between the anti-inflammatory capacity of HDLs and incident CV events was recently observed in a study of individuals from the general population cohort, independently of both HDL-C and CEC
[64][139].