2. Impact of CF-LVAD on Gastrointestinal Vasculature and GIB
VWF plays a vital role in pathophysiology of GIB (
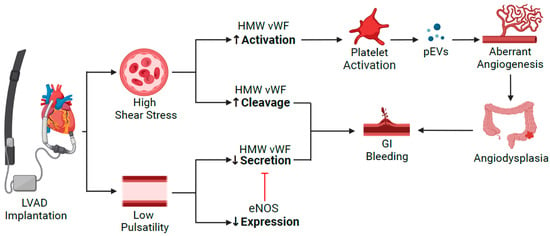
Figure 12). The attenuated pulsatility in CF-LVAD seems to result in direct inhibition of VWF secretion by endothelial cells and indirect inhibition by reducing endothelial NO secretion that leads to negative-feedback inhibition of VWF secretion
[7][30].
Figure 12. Pathophysiology of GIB in patients supported with CF-LVAD. VWF deficiency is the common pathway for development of angiodysplasia due to the high shear stress and continuous flow. eNOS: endothelial nitric oxide synthase; GI: gastrointestinal; HMW VWF: high molecular weight von Willebrand Factor; pEVs: platelet-derived extracellular vesicles.
VWF deficiency in the context of CF-LVAD occurs either through pump shear-induced VWF activation with subsequent exposure of the ADAMTS-13 cleavage site for enzymatic degradation or by shear stress-induced fragmentation of VWF into dysfunctional small fragments, independent of ADAMTS-13
[4][8][9][10][11][4,31,32,33,34]. The net effect of decreased production and increased degradation of VWF culminates in diminished VWF-dependent platelet aggregation.
The lack of physiological pulsatility in CF-LVAD-implanted patients results in GI mucosal hypoxia with subsequent sympathetic activation and release of angiogenesis factors such as VEGF and angiopoietin 2. These factors cause smooth muscle relaxation and dilation of the mucosal veins, which results in arteriovenous malformations (AVM) and angiodysplasias
[12][13][35,36]. VWF is thought to be a negative regulator of angiogenesis by reducing VEGF-2-dependent proliferation of endothelial cells by extracellular binding to integrin αvβ3
[14][37]. The loss of VWF with subsequent defective Weibel Palade body formation promotes angiodysplasia due to ineffective intracellular storage and release of angiopoietin-2
[14][15][37,38].
Notably, a contemporary study has suggested that VWF becomes hyper-adhesive in CF-LVAD patients rather than being excessively cleaved
[16][39]. The hyper-adhesive VWF was shown to activate platelets and produce platelet-derived extracellular vesicles with subsequent local concentration of VEGF and development of aberrant angiogenesis
[16][39].
2.1. Clinical Perspective
Despite the beneficial role of LVADs in patients with advanced heart failure, GIB is one of the major complications in this patient population
[17][18][40,41]. The reported incidence of GIB post-CF-LVAD implantation ranges from 21% to 31%
[19][20][42,43]. Multiple studies of GIB in LVADs reported that the majority of cases had therapeutic or subtherapeutic INR levels at the time of bleeding
[20][43]. Predictors of GIB include older age, redo sternotomy, preoperative inotrope use, elevated preoperative creatinine, RV failure, and concomitant antiplatelet and anticoagulant use
[19][42].
Upper GIB seems to be the main location of bleeding in LVAD patients with AVM, with angiodysplasia being the most common culprit
[20][21][43,44]. In a pooled analysis of 1087 patients, the mean duration from CF-LVAD implantation to first bleeding event was 54 days, and anemia was the most common presentation, followed by melena
[22][45].
The occurrence of GIB is associated with increased morbidity and mortality. Moreover, the need for repeated blood transfusions increases alloimmunization risk, which may limit HT offers
[23][46]. This prompts the need to develop treatment strategies to prevent GIB.
2.2. Interventions and Medications to Reduce GIB in CF-LVAD-Implanted Patients
2.2.1. Pump Design
The HM 3 device is recognized as a fully magnetically levitated device that has the potential to reduce shear stress, and it provides artificial pulsatility. Notwithstanding, these design changes did not lead to lower GIB when compared to HM 2 in the momentum trial
[24][47]. Netuka et al. documented an 18% decrease in VWF with the HM 3 device, compared with a 46% to 73% reduction with the HM II device, after 45 days of support, with no measurable differences in ADAMTS-13 activity levels
[25][48]. This suggests that the HM 3 device may still induce mechanical shear stress adequate to disturb VWF homeostasis. Furthermore, it seems that the HM 3 device likely provides an arterial pulsatility below the physiologic minimum level needed to reduce bleeding events.
The markedly lower pump thrombosis rates with newer CF-LVADs, particularly the HM 3 LVAD, has prompted discussion regarding the potential for avoidance of antiplatelet therapy to reduce bleeding risk based on observation data showing lower risk of re-bleeding without increase in thrombotic complications after discontinuation of aspirin in HM2 and HM3 LVAD patients
[26][27][28][29][49,50,51,52]. However, the feasibility of such a strategy may be device-specific, as an Aspirin dose of 81 mg daily instead of 325 mg daily was associated with increased risk of thrombosis for the HeartWare HVAD pump, which was not the case for the HM3 and HM 2 devices
[30][31][32][33][53,54,55,56].
2.2.2. Medical Management
There is limited data regarding the potential benefit of pharmacological interventions, most of which comes from observational studies in patients with recurrent or refractory LVAD-related GIB.
It has been hypothesized that angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs) may reduce angiogenesis by inhibiting angiotensin II-related activation of the transforming growth factor beta (TGF-β) and VEGF pathways. A recent systematic review and meta-analysis by Kittipibul et al. found that retrospective data from 3 studies
[34][35][36][57,58,59] with a total of 619 CF-LVAD patients showed ACEi/ARB use was associated with a decreased incidence of overall GIB
[37][60]. Interestingly, while there was a trend towards reduced odds of AVM-related GIB with ACEI/ARB use, it was not statistically significant as the largest study by Shultz et al. with 377 patients found no significant difference in AVM-related GIB rates by ACEi/ARB usage
[35][58]. The protective effect seems to be seen with a dose threshold of >5 mg daily lisinopril equivalence rather than being dose-dependent
[36][59] and seems independent of BP effect
[34][57]. Unfortunately, the definitions of ACEI/ARB usage and GIB events vary amongst these studies. Furthermore, there is conflicting data showing no significant association between GIB risk and ACEi/ARB use in a retrospective analysis using data from 13,732 patients in the INTERMACS registry, about 52% of whom were on ACEi/ARB
[38][61]. Interestingly, this analysis showed a lower risk of GIB in patients on beta blockers
[38][61], which was not the case in the smaller study by Houston et al.
[34][57].
The anti-VEGF monoclonal antibody, bevacizumab, was well tolerated and markedly reduced the need for transfusions, endoscopies, and GIB-related hospitalizations in a small pilot study involving five HM II LVAD patients with refractory angiodysplasia-related GIB over a median follow-up period of 22 months
[39][62].
The somatostatin analog, octreotide, lowers portal pressures by splanchnic vasoconstriction and downregulates VEGF and basic fibroblast growth factor to inhibit angiogenesis, and it has been used in variceal bleeding as well as non-variceal angiodysplasia-related GIB
[40][63]. It is well tolerated, with most of the available data from small observational studies showing some potential benefit in reducing GIB recurrence in CF-LVAD patients
[40][41][42][43][44][45][46][47][63,64,65,66,67,68,69,70].
The data regarding digoxin’s potential role in LVAD-related GIB management is inconclusive. There are a few retrospective studies linking digoxin use to significant reduction in all-cause GIB, particularly in angiodysplasia-related GIB in CF-LVAD patients
[48][49][50][71,72,73]. The proposed mechanism is suppression of hypoxia-inducible factor-1 α (HIF-1α), a mediator of angiopoietin-2-induced angiodysplasia
[48][71]. However, a large retrospective analysis using the INTERMACS database by Jennings et al. in 2020, with over 2000 CF-LVAD patients on digoxin, found no association between LVAD-related GIB rates and digoxin use
[38][61].
There is very limited data regarding the use of hormone therapy in CF-LVAD-related GIB. A small single-center proof-of-concept retrospective observational study has suggested significant reduction in GIB-related transfusions and hospitalizations with danazol use
[51][74]. There is conflicting data regarding estrogen-based hormone therapy for AVM-related GIB prevention, and the potential for increased thromboembolic risk poses reservations.
Thalidomide is thought to downregulate HIF-1α expression and inhibit VEGF and basic fibroblast factor
[52][53][54][55][75,76,77,78]. Its antiangiogenic properties have shown some promise in refractory angiodysplasia-related GIB, including in LVAD patients
[55][56][57][58][59][78,79,80,81,82]. The largest retrospective study to date showed that thalidomide use in 17 CF-LVAD patients with angiodysplasia-related GIB was found to significantly reduce the risk of rebleed, median number of GIBs per year, and transfusion requirements per year while on thalidomide versus while off thalidomide (before initiation)
[55][78]. Adverse event rate was 59%, albeit with dose reduction resolving symptoms in most patients without increased GIB
[55][78]. Barriers to its use include high incidence of adverse effects which seem to be dose related, with unclear minimal effective dose, and the need for provider and pharmacist enrollment in the THALOMID Risk Evaluation and Mitigation Strategy (REMS) program to prescribe thalidomide due to teratogenicity.
Desmopressin is a vasopressin analog currently used to treat Hemophilia A and von Willibrand disease. It shortens bleeding time and improves hemostasis by increasing VWF and factor VIII levels, making it an attractive potential therapy for the acquired VWF deficiency implicated in LVAD-related GIB. In one case report, desmopressin prevented rebleeding for 6 months in one HM II LVAD patient with refractory GIB, despite holding antithrombotic therapies and starting octreotide
[60][83]. The data is inadequate to provide a recommendation, as further studies are needed to determine efficacy and safety given potential for hyponatremia, fluid retention, and thrombosis.