Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nikolaos Nenadis and Version 2 by Camila Xu.
Theoretical examination of the antioxidant activity of the individual or selected compounds identified in a matrix can be a green mid-tool for their prioritization regarding antioxidant behavior before seeking experimental in vitro or in vivo data.
- antioxidant activity
- theoretical predictions
- phenolic compounds
- olive tree products
- olive tree by-products
- oleuropein
- hydroxytyrosol
- tyrosol
- secoiridoids
- density functional theory
1. Introduction
The interest in natural compounds that exhibit antioxidant activity in food matrices [1] is extended also to the benefits such compounds can exert on humans or animal species after consumption [2][3][2,3]. For example, the high oxidative stability of virgin olive oil (VOO) at first was attributed to its rich in monounsaturated fatty acids character and then to the critical role of certain groups of intrinsic antioxidants such as the phenolic compounds present in the polar fraction of the oil. For more than two decades, the interest has been focused on the in vivo properties of the secoiridoids oleuropein, ligstroside, and related derivatives, which can be found not only in the oil but also in other edible products of the olive tree or can be isolated from by-products of the olive industry [4][5][4,5].
Antioxidants, in general, exhibit their activity even when present in minute concentrations. The phenolic-type antioxidants are considered primary antioxidants because they react directly with reactive oxygen and nitrogen species via hydrogen atom and/or electron donation and have gained the interest of food, medical, pharmaceutical, and cosmeceutical researchers all over the world [6].
2. Theoretical Prediction Strategies
As a result of advances in computer science and software engineering, it is feasible to accomplish theoretical calculations for compounds of medium or even larger size in a reasonable amount of time, making them suitable even for routine studies. The overall number of publications regarding antioxidants that involve theoretical calculations is steadily increasing, but the latter have not found their place in the agriculture and food sector yet. This is indicated by the bibliometrics shown in Figure 1.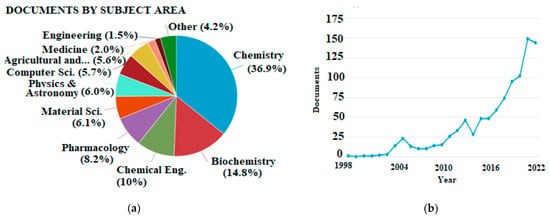
Figure 1. (a) Publications categorized by subject area and (b) total publications per year appearing in Scopus database using “density functional theory” and “antioxidant” as keywords (accessed 21 November 2022, total documents, 995).
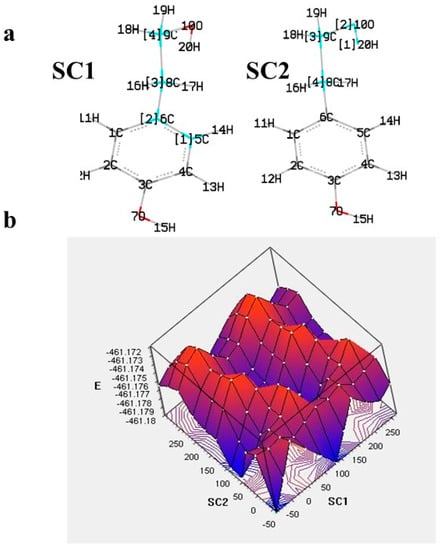
Table 1. Common molecular indices related to the radical scavenging potential of compounds and the formulae for their calculation.
| Molecular Index | Formula * |
|---|---|
| Bond dissociation enthalpy (BDE) | Hr + Hh − Hp |
| Adiabatic ionization potential (IP) | Ecr − Ep |
| Proton dissociation enthalpy (PDE) | Hr + Hpr − Hcr |
| Proton affinity (PA) | Ha + Hpr − Hp |
| Electron transfer energy (ETE) ** | Er − Ea |
Where * Hr: enthalpy of phenoxy radical, Hh: enthalpy of the H-atom, Hp: enthalpy of the parent molecule, Ecr: energy of the cation radical, Ep: energy of the parent molecule, Hpr: enthalpy of the proton, Hcr: enthalpy of the cation radical, Ha: enthalpy of the anion, Er: energy of the phenoxy radical, Ea: energy of the anion. (** In some publications, the enthalpies are considered, including also in the formula the enthalpy of the electron).
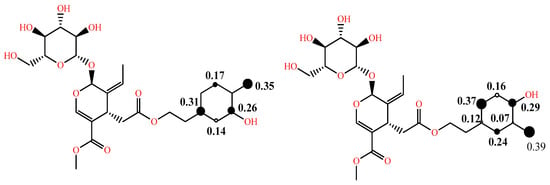
In addition to the above, in some studies, to comment on the reactivity and the dominant mechanism, the radical species of interest are also considered, and their BDE and electron affinity (EA) values are computed as well [22][29]. As the effectiveness of a radical scavenger is not only related to the ease of hydrogen atom and/or electron donation, but also to the stability of the phenoxy radical formed through intramolecular bonding or extension of conjugation, the spin distribution can be obtained and, particularly, the value of the localized spin in the oxygen of the corresponding radical, which can be a useful index of radical stability. The latter is illustrated in Figure 3 for the two phenoxy radicals of oleuropein [23][30]. Due to the lack of extended conjugation, the electron spin is delocalized only in the aromatic ring. Furthermore, the radical formed from the p-OH group is more stable than that from the m-OH group, as is evident by the spin value in the oxygen of the corresponding radicals (0.35 vs. 0.39).
 When selecting to work from a thermochemical point of view, the calculation of the Gibbs free energy (ΔG) value is the most appropriate index to examine a specific reaction pathway. According to the equation ΔG = ΔH − Δ(TS), its calculation, except for temperature (T), also includes the entropy (S) of the system that is influenced by the mechanism governing the reaction. The approach is considered to offer more valuable information compared to that obtained from the calculation of indices of intrinsic reactivity, as both the reactivity of the antioxidant and that of a particular free radical species are co-evaluated. The latter is important, considering that the various free radicals to be scavenged differ in reactivity. A drawback, as highlighted by Galano and Alvarez-Idaboy [15][22], is that when using such a strategy, kinetics is not taken into consideration, and in some cases, opposite trends may be observed due to deviation from pertinence to the Bell–Evans–Polanyi principle, i.e., the usual linear relationship between the activation energy and enthalpy of reaction. Furthermore, it should be stressed that to make feasible the calculation, small-size radicals are usually selected, such as HO●, O2●−, CH3OO•, and HOO• [24][25][31,32].
When a kinetic study is designed for a biological antioxidant, the starting point of consideration is that, according to the definition of Halliwell et al. [26][33], a compound should react faster with the oxidizing species than the substrate to be considered as a potentially efficient antioxidant. The advantages of kinetic approaches, according to Galano and Alvarez-Idaboy [15][22], are the following:
When selecting to work from a thermochemical point of view, the calculation of the Gibbs free energy (ΔG) value is the most appropriate index to examine a specific reaction pathway. According to the equation ΔG = ΔH − Δ(TS), its calculation, except for temperature (T), also includes the entropy (S) of the system that is influenced by the mechanism governing the reaction. The approach is considered to offer more valuable information compared to that obtained from the calculation of indices of intrinsic reactivity, as both the reactivity of the antioxidant and that of a particular free radical species are co-evaluated. The latter is important, considering that the various free radicals to be scavenged differ in reactivity. A drawback, as highlighted by Galano and Alvarez-Idaboy [15][22], is that when using such a strategy, kinetics is not taken into consideration, and in some cases, opposite trends may be observed due to deviation from pertinence to the Bell–Evans–Polanyi principle, i.e., the usual linear relationship between the activation energy and enthalpy of reaction. Furthermore, it should be stressed that to make feasible the calculation, small-size radicals are usually selected, such as HO●, O2●−, CH3OO•, and HOO• [24][25][31,32].
When a kinetic study is designed for a biological antioxidant, the starting point of consideration is that, according to the definition of Halliwell et al. [26][33], a compound should react faster with the oxidizing species than the substrate to be considered as a potentially efficient antioxidant. The advantages of kinetic approaches, according to Galano and Alvarez-Idaboy [15][22], are the following:

-
Consideration of tunneling effects for reactions involving small particles;
-
Contribution of different mechanisms of reaction, and reaction sites, to the whole activity of the examined compound;
-
Inclusion of reactions that do not fulfill the Bell–Evans–Polanyi principle;
-
Incorporation of the pH influence on the reactivity of antioxidants bearing ionizable groups such as acids, considering the molar fraction of different forms present when the pH value is different;
-
Consideration of single electron transfer reactions situated in the inverted region of the Marcus parabola.
Table 2. Branching ratios (Γ) for the most important mechanisms and pathways involving hydroxytyrosol and methoxy radical in pentyl ethanoate and water (data abstracted from Table 2 of cited reference [27][34] and adjusted properly).
| Structure and Numbering | Pentyl Ethanoate | Water |
|---|---|---|
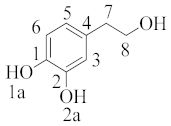 hydroxytyrosol |
HAT(1a) = 61.53 | HAT(1a) = 49.69 |
| HAT(2a) = 37.69 | HAT(2a) = 49.69 | |
| HAT(7) = 0.06 | HAT(7) = 0.03 | |
| HAT(8) = 0.14 | HAT(8) = 0.10 | |
| RAF*(1) = 0.55 | RAF(1) = 0.20 | |
| RAF(2) = 0.00 | RAF(2) = 0.12 | |
| RAF(3) = 0.00 | RAF(3) = 0.01 | |
| RAF(4) = 0.03 | RAF(4) = 0.16 | |
| RAF(5) = 0.00 | RAF(5) = 0.01 | |
| RAF(6) = 0.00 | RAF(6) = 0.00 |
* RAF = radical adduct formation.
