Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Jessie Wu and Version 3 by Jessie Wu.
Congo-red (CR) is an azo dye with the molecular formula of C32H22N6Na2O6S2 and molecular weight = 696.68 g mol−1. Paul Bottinger discovered CR as the first direct dye in 1884. It is an anionic di-azo dye (contains two groups -N=N-) composed of a sodium salt of benzidinediazo-bis-1-naphthylamine-4-sulfonic acid, known by common names such as CR 4B, C.I. 22120, Cotton red B, Cotton red C, Direct red 28, Cosmos red, Direct red Y, and Direct red R.
- dyes
- Congo red
- toxicity
1. Congo Red
Congo-red (CR) contains two azo (-N=N-) chromophores and acidic auxochromes (-SO3H) linked with the benzene structures [1][2]. CR is also called acidic diazo dye. A molecule of CR is linearly symmetrical, with a hydrophobic center consisting of two phenyl rings connected via di-azo linkage [3]. The phenyl rings are connected to two charged terminal naphthalene moieties containing sulfonic and amino groups [4]. The chemical structure of CR is shown in Figure 1, and it is made by mixing two molecules of naphthenic acid with tetrazotized benzidine [5]. A blue dye is obtained and converted into red disodium salt while salting out with sodium chloride.
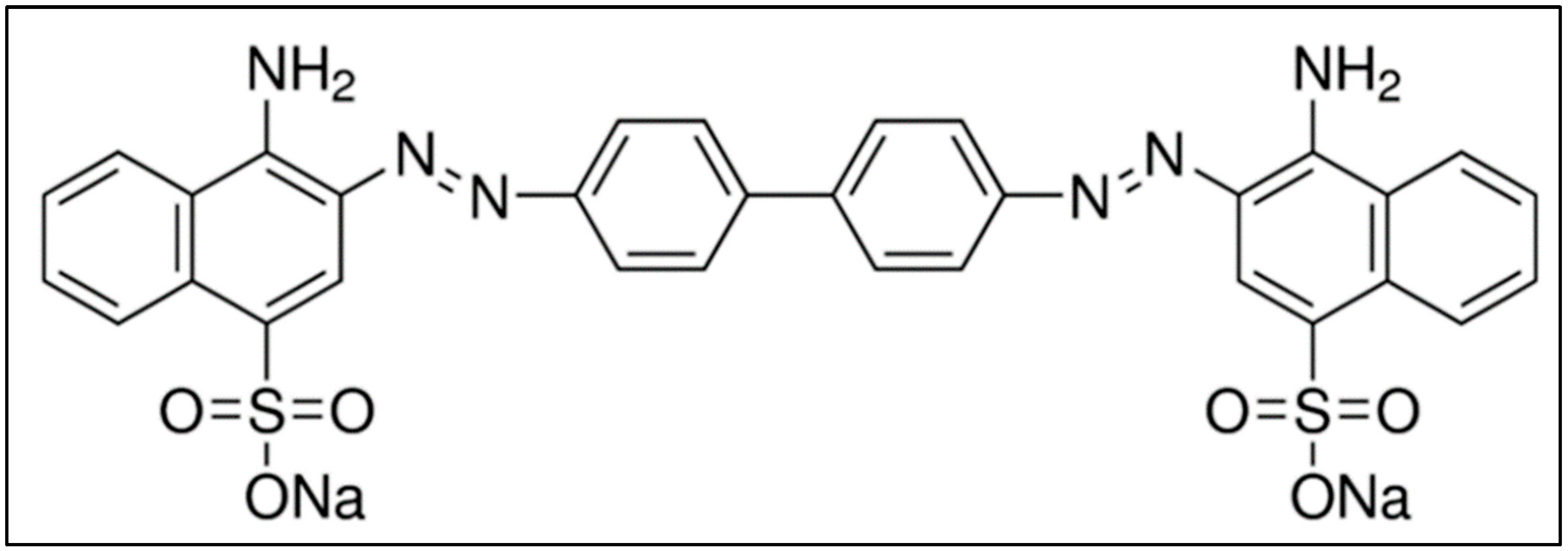
Figure 1. Molecular structure of CR. Adapted from the literature [5].
CR’s color index number ranges from 2000 to 29,999. CR is also called acidic di-azo dye and is water soluble. CR gives a characteristic color in an aqueous solution due to the presence of an azo structure. It appears red in the basic medium and blue in the acidic medium because it is a di-azo dye. CR can form an amine constituent such as benzidine on the cleavage of its azo groups. Benzidine is a common carcinogen, so CR is included under the category of banned azo dyes [3]. Although CR finds practical applications in many industries like textile, cosmetics, pigment, leather, food, pharmaceutical, pulp, and paper, its extensive use also leads to industrial pollution. Figure 2 illustrates the major sources of CR pollution in the environment [6].
Figure 2. Major sources of CR contamination in the environment.
2. Toxic Effect of Congo Red on Humans
CR adversely affects the human body leading to various diseases, which can be fatal, when its concentration is high from being cytotoxic (genotoxic, hemotoxic, and neurotoxic), carcinogenic, and mutagenic [7]. Among the organs, it affects the eyes, skin, respiratory, and reproductive systems. Benzidine (its toxic metabolite) causes an allergic reaction and is a carcinogenic product. Benzidine is a bladder carcinogen and binds covalently to cellular macromolecules leading to activity inhibition. In animal experiments, benzidine-based dyes produce hepatocarcinoma, splenic sarcoma, nuclear abnormalities, and chromosomal errors in mammalian cells [8]. CR can cause platelet aggregation, thrombocytopenia, and disseminated micro-embolism by lowering blood protein content [4].
As reported earlier, CR has been known to cause an inhibitory effect on the activities of two enzymes: aspartate aminotransferase and alanine aminotransferase, mainly found in the liver. During tissue traumas such as cellular necrosis and cell growth, both enzymes are dispersed intracellularly and escape into the bloodstream. Occasionally, when tissue damage occurs, the activities of the enzymes are raised [9].
As reported, CR interacts covalently with proteins in organs other than the liver. One of the reasons could be that the function of enzymes is bound to be influenced since they are proteins. Benzidine, the metabolic product of CR, is mainly behind enzyme activity inhibition. Moreover, CR was also found to lower glutathione levels in rabbit and rat serum [9]. According to a study, CR in high dilution hastens the coagulation of cats’ blood, whereas higher dye concentrations may slow clotting and retain the blood in a fluid state [10].

Figure 2. Major sources of CR contamination in the environment.
3. The Toxic Effect of Congo Red on Aquatic Species
To be mutagenic, large proportions of azo dyes demand chemical activation, such as azo link reduction and cleavage to aromatic amines (AA). The biological toxicities of these dyes were seen for various pathogens species such as bacterium (Vibrio fischeri, V. fischeri), microalga (Selenastrum capricornutum, S. capricornutum), ciliate (Tetrahymena pyriformis, T. pyriformis) [11], Cladoceran (Daphnia magna, D. magna and Ceriodaphnia rigaudi, C. rigaudi), and Zebrafish (Danio rerio. Pseudokirchneriella subcapitata, D. rerio. P. subcapitata) [12]. The level of toxicity ranges from species to species.
Bacterial, algal, protozoan, Ames, and flash bioluminescence tests were employed to know the dyes’ toxicity [11]. Toxicological tests supplement cytotoxicity data, including morphological and physiological assessments.
3.1. Bacterial Test
CR dye is known to be carcinogenic and harms the intestinal bacteria involved in digesting it [13]. Apart from the positive contribution of intestinal bacteria to our health, it can also pose a negative effect. The chemical reduction and breaking of azo bonds by azoreductase, which is found in bacteria, cause the toxicity of azo dye. Rhodococcus rhodochrus (R. rhodochrus) is one of the bacteria that make up our intestinal microbiota, capable of reduction and cleavage of a large number of organic compounds [13][14]. The aerobic Gram-positive bacteria can also metabolize CR. Despite the R. rhodochrous metabolites inserted into the bacterial culture, they cause a toxic effect on bacterial growth. The higher the dye metabolized, the higher the toxicity of the dye to bacterial growth [13].
3.2. Algal Test
The algal test is considered to be the most sensitive to dyes compared with other tests. Nevertheless, CR toxicity can be diagnosed with a variety of biochemical tests involving the zebrafish (D. rerio), the microalga (P. subcapitata), and the cladocerans (D. magna and C. rigaudi).
P. subcapitata was the most susceptible to CR, with half maximal inhibitory concentration (IC50) being 3.11 mg/L [12]. P. subcapitata growth was affected at all concentration ranges. With increasing CR concentrations, the number of photosynthetic pigments, chlorophyll A and β, carotenoid content per cell increased. The impact of CR on macromolecule content (proteins, carbs, and fats) was also reported to be increased. The cladocerans (D. magna and C. rigaudi) survival decreased as CR concentration increased. D. magna, on the other hand, was more resistant to CR dye than C. dubia. Several consequences of azo dyes were observed in zebrafish embryos, ranging from malformations to hatching failures. Zebrafish embryos are not acutely harmed by CR dye; it produced sublethal effects. Among those effects were edema in the yolk sac, skeletal abnormalities, and delayed or precluded hatching. Hernández-Zamora and Martínez-Jerónimo [12] reported the reduction in the growth rate, photosynthesis, and respiration that CR has caused in green microalgae species Chlorella vulgaris (C. vulgaris). The growth rate is reduced due to a decrease in metabolic activity. The metabolic rate (photosynthesis and respiration) was suppressed at all CR concentrations. The photosynthetic and respiratory processes were reduced by 84 and 98 percent and 76 and 96 percent, respectively, at 5 and 25 mg/L concentrations. The cells continue to develop slower even when the photosynthetic and respiratory rates drop dramatically. Photosystem II (PSII) changes were found in response to CR at 10 to 25 mg/L. The donor site of PSII is notably affected in terms of photosynthetic activity. Non-photochemical thermal dissipation pathways increase due to the decreased ability to absorb and utilize quantum energy.
3.3. Flash Bioluminescence Test
A flash bioluminescence test was used to determine the acute toxicity of the dark-colored compound. However, the sensitivity of the flash bioluminescence test was found to be low for azo dyes such as CR. The EC50 value of CR was found to be 1623 mg/L with V. fischeri bacterium [11].
3.4. Ames Test
The Ames test measured the dyes’ genetic toxicity with and without metabolic activation. It was found to be more sensitive than the flash bioluminescence test. The EC50 value of CR was found to be 4.8 ± 1.0 mg/L with microalga, S. capricornutum [11].
3.5. Protozoan Tests
Protozoan tests were also used to assess the toxicological potential of azo dyes in aquatic environments using ciliated protist T. pyriformis. A dose of 500 mg/L of dyes was used. The test typically included a grazing experiment and morphometric analysis (cell area and cell width/length (W/L) ratio), as well as a protocol developed for measuring population growth impairment and generation time [11]. After a 24-h exposure duration, CR was observed to modify the cell area value [11] drastically.
3.6. Genetic Toxicity Test
To detect substitution mutations and shift mutations, auxotrophic strains Salmonella typhimurium (S. typhimurium), His TA100 and YG1042 strains, and TA98 and YG1041 were used, respectively. The dye concentration per agar plate ranges from 50 to 400 µg. Including sulfonic groups in the dye molecule, which can reduce the mutagenic effect, may explain the negative results reported with Remazol Brilliant Blue R and CR [11].
4. The Toxic Effect of Congo Red on Plants
The severity of azo dye’s toxicity (genotoxic and cytotoxic) is also visible in aquatic plant life. Azo dye, when mixed with water, reduces the penetration of light which alters the photosynthesis process, and, thus, the ecosystem is negatively affected [15]. When released into water, it not only raises the pH, biological oxygen demand (BOD), and chemical oxygen demand (COD) of the water but also disrupts the environment’s organic-inorganic chemical equilibrium. The biotic content of water is affected by these organic-inorganic chemical concentrations in the environment [16].
As reported in earlier literature [17], CR is proven to be toxic for the aquatic plant species, Lemna minor (LM), point of view physiological and cytogenetic. According to reports, altering the CR concentrations has several negative consequences on the LM plant including root growth, fresh mass at 5 parts per million (ppm), and reduced total frond surface. Above 2500 ppm, CR inhibits LM plant growth, decreases chlorophyll A content, and increases carotenoid content. Above 1000 ppm, it also shows a considerable decline in PSII efficiency, a fall in mitotic indices at 5 and 1000 ppm dye, and zero at 5000 ppm. Additionally, at 5 and 1000 ppm dye, an increase in the figure of chromosomal abnormalities was observed, and a 56 percent decontamination of the growing medium has been reported at 250 ppm. Another study reported CR toxic effects on the LM plant [18]. At all CR values more than 1000 ppm, this research found an increase in chlorophyll B. LM plants underwent certain stress by CR due to elevated carotenoid amounts and reduced chlorophyll fluorescence values. This supposition is further sustained by the higher phenolic contents in treated plants, as it is known that such parameters may reflect abiotic stress levels. Two other azo dyes (acid scarlet GR or acid red B) have been found to have adverse effects on two plant species, Medicago sativa L (M. sativa L) and Sesbania cannabina Pers (S. cannabina pers). These plant species were resistant to azo dyes at low concentrations. Germination rate and root elongation were not considerably reduced in Medicago sativa L. plants at concentrations below 1 g/L, but significantly reduced at 5 g/L. Even though the azo dyes were used at modest concentrations, root elongation in S. cannabina pers was reduced [19].
References
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Inamuddin; Asiri, A.M. Nano-engineered Adsorbent for the Removal of Dyes from Water: A Review. Curr. Anal. Chem. 2020, 16, 14–40.
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive amputation of hazardous azo dye Congo red from wastewater: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 14810–14853.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 11313, Congo Red. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Congo-red (accessed on 5 February 2023).
- D’Souza, E.; Fulke, A.B.; Mulani, N.; Ram, A.; Asodekar, M.; Narkhede, N.; Gajbhiye, S.N. Decolorization of Congo red mediated by marine Alcaligenes species isolated from Indian West coast sediments. Environ. Earth Sci. 2017, 76, 721.
- Clavijo, C.; Osma, J.F. Functionalized leather: A novel and effective hazardous solid waste adsorbent for the removal of the diazo dye congo red from aqueous solution. Water 2019, 11, 1906.
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531.
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical Agents and Related Occupations. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100F. Dyes metabolized to benzidine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304402/ (accessed on 7 March 2023).
- Obi, F.O.; Maduka, H.C.C.; Zubairu, I. Assessment of congo red-induced liver damage by selected serum transaminase levels. J. Med. Sci. 2003, 3, 157–162.
- Macht, D.I. Experimental studies on heparin and its influence on toxicity of digitaloids, congo red, cobra venom and other drugs. Ann. Intern. Med. 1943, 18, 772–791.
- Novotný, Č.; Dias, N.; Kapanen, A.; Malachová, K.; Vándrovcová, M.; Itävaara, M.; Lima, N. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo-and anthraquinone dyes. Chemosphere 2006, 63, 1436–1442.
- Hernández-Zamora, M.; Martínez-Jerónimo, F. Congo red dye diversely affects organisms of different trophic levels: A comparative study with microalgae, cladocerans, and zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2019, 26, 11743–11755.
- Smith, R.; John, G. Azo Dye Toxicity: A measure of toxic effect metabolized azo dyes have on the body. Biol. Chem. Eng. 2016, 2, 1–4.
- Busch, H.; Hagedoorn, P.L.; Hanefeld, U. Rhodococcus as A Versatile Biocatalyst in Organic Synthesis. Int. J. Mol. Sci. 2019, 20, 4787.
- Imran, M.; Shaharoona, B.; Crowley, D.; Khalid, A.; Hussain, S.; Arshad, M. The stability of textile azo dyes in soil and their impact on microbial phospholipid fatty acid profiles. Ecotoxicol. Environ. Saf. 2015, 120, 163–168.
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of synthetic azo dyes of textile industry: A sustainable approach using microbial enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131.
- Lobiuc, A.; Olaru, S.; Hancu, E.I.; Costica, N.; Fortuna, M.E.; Zamfirache, M.M.; Constantinescu, G. Toxicity and removal of direct red 28 diazo dye in living polymeric systems. Rev. Chim. 2018, 69, 1628–1635.
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802.
- Zhou, X.; Xiang, X. Effect of different plants on azo-dye wastewater biodecolorization. Procedia Environ. Sci. 2013, 18, 540–546.
More
